Genotoxic and Hypogonadism Effect of Triclosan Treatment and the Mitigating Effect of Vitamin E in Male Albino Mice
2 Faculty of Science, Taif University, Al-Hawyeia, KSA
Citation: Nahed AH, Hamida H. Genotoxic and Hypogonadism Effect of Triclosan Treatment and the Mitigating Effect of Vitamin E in Male Albino Mice. J Basic Clin Pharma 2017;8:200-204.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
The widely used antimicrobial compound, triclosan (TCS), causes various harmful impacts to human. The present study was aimed to evaluate the genotoxic and antiandrogenic potential of TCS represented by DNA damage (Comet assay), histopathological and testicular hormonal evaluations. Moreover, the study was aimed to assess the protector role of vitamin E (Vit E) pre-treatment in testes of albino male mice. TCS was injected IP at dose level (15 mg/kg) for 2 consecutive days. Other group was orally administrated with Vit E (50 mg/kg) just before TCS injection. Mice were sacrificed after 24 hr from the last treatment. Testicular tissues were used for molecular, histopathological evaluations and different testicular hormones (testosterone, FSH and LH) determination. The present study reports the genotoxic effect of TCS because of a significant increase in tail length, %DNA in tail and tail moment for TCS group in comparison with the negative control group in comet assay. Moreover, TCS treatment causes damage to testis architectures represented by the separated seminiferous tubules, pyknotic spermatocytes, vacuolization in seminiferous epithelium due to loss of Sertoli cells and hypospermatogenesis. In addition, TCS treatment significantly decreases testicular hormones (testosterone, FSH and LH) in comparison to negative control group. However, Vit E pre-oral administration reverses the toxic effect of TCS. In conclusion, the present study reports the genotoxic and antiandrogenic effect of TCS in male mice and the ameliorative effect of Vit E pre-oral administration as a result of its ROS scavenger properties
Keywords
Triclosan, vitamin E, comet assay, FSH, LH, testosterone
Introduction
Several antimicrobial agents and preservatives (soaps, shampoos, detergents, disinfectants, cosmetics and pharmaceutical products) are commonly used in the personal care products.[1-3] Different parts of our body like blood, milk, and various organs and tissues are the target of those chemical accumulations due to their continuous use with detectable concentrations.[1,4,5] Triclosan (TCS) is one of those antimicrobial agents that widely used as preservative in toothpastes, soaps, shampoos, and cosmetics [6] and reported to be a highly toxic chemical for aquatic flora and fauna [7] and thus has been included in the probable list of endocrine disruptors because of its resemblance with known non-steroidal estrogens or its mimetic.
Furthermore, heat and ultraviolet irradiation convert TCS and its chlorinated derivatives to various chlorinateddibenzo-p-dioxins that might be harmful for different biological systems.[8-10] The mode of action of TCS as an Endocrine-disrupting chemical (EDC) is controversial, in which TCS exposure in Japanese medakafry (Oryziaslatipes) for fourteen days showed a weak androgenic effect.[11] Another study reported that TCS metabolite may be a weak estrogenic compound with the potential to induce vitellogenin while decreasing the hatchability, as well as delaying the hatching in females.[12] TCS has also been reported to inhibit testosterone-induced transcriptional activity as it functions as an anti-androgen agent.[13] Exposure of TCS to the human may be a consequence of its presence in the cosmetics and other human use products that could be absorbed mainly across the skin or through the gastrointestinal tract.[14]
Environmental contaminants distribute the pro-oxidant/antioxidant balance of testicular cells that in turn affects their functions, accompanied by downstream pathways such as apoptosis.[15] In turn this increases reactive oxygen species (ROS) levels and apoptosis and affects the normal functioning of the testis tissue. Therefore, there is a great demand to use a good antioxidant to reduce this deleterious effect.
The present study was aimed to evaluate the histopathological, DNA damage and testicular hormonal evaluations induced by TCS treatment. Moreover, the study was aimed to assess the protector role of vitamin E pre-treatment in testes of albino male mice.
Materials and Methods
Animals
Twenty-five albino male mice (Mus musculus; 8-10 weeks old; 26-30 g body weight) were obtained from the animal house of the King Fahad Center for Medical Research, King Abdul-Aziz University in Jeddah. We have followed the European Community Directive (86/609/EEC) and National Rules on Animal Care.
Tested drugs
1. Triclosan (TCS) (Molekula) was dissolved in corn oil and injected intraperitoneally (i.p.) daily at 15 mg/kg for 2 consecutive days.[16]
2. Vitamin E (Vit E) (α-tocopherol acetate, Sigma) was dissolved in corn oil and orally was administrated daily for 2 consecutive days at dose level of 50 mg/kg.[17]
Treatment Schedule
Group 1: Negative control group, untreated mice. Group 2: Vehicle group, mice were injected i.p. with 50 μL corn oil. Group 3: Vitamin E (Vit E) group, mice were administrated orally with 50 μL Vit E in corn oil (50 mg/kg). Group 4: Triclosan (TCS) group, mice were injected i.p. with 50 μL TCS (15 mg/kg). Group 5: Vitamin E+triclosan (Vit E+TCS), mice were orally administrated with 50 μL Vit E in corn oil followed by i.p. injection of 50 μL TCS (15 mg/kg+50 mg/kg).
Animals were killed by cervical dislocation and testicular tissues were used for further assays.
Molecular Evaluation
Comet assay
Frosted microscopic slides were dipped into 1.0% hot normal melting point agarose with the remove of excess agarose from the underside of the slide. Testes tissues were homogenized in cold Hank`s Balanced Salt Solutions and only 10 μL of homogenate was mixed with 65 μL of low melting point agarose (0.5%) at 37°C, and was spread on the frosted slides. Then the slides were left in lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mMTris, NaOH to pH 10.0, 1% Triton-100 and 10% DMSO) for 2 hours at 4°C. The slides were left in electrophoresis buffer (NaOH, TE buffer) for 20 mins and then electrophoresed at a constant current of 300 mA, for 35 mins. After complete electrophoresis, the slides were neutralized using Tris-HCl buffer (5 mins for three washes) at pH 7.5, followed by cold ethyl alcohol for 10 mins and then left to dry overnight. The slides were stained by using 80 μL ethidium bromide (20 μg/ mL) for 20 mins.[18] The slides were covered and viewed under an epifluorescence microscope (Zeiss epifluoresent) with an attached CCD camera. Images were saved as electronic files and for each sample, 50 isolated comets were selected randomly and measured for comet tail length, %DNA in tail and tail moment using COMETSCORE software based on the definition.[19]
Histopathological Evaluation
Testes were removed and fixed in 10% neutral buffer formalin, washed with tap water, dehydrated in a series of ethyl alcohols, then cleared in xylene, and finally embedded in 60°C paraffin wax to obtain block. Blocks were cut at 5 microns using a microtome. Testis sections were stained using Hematoxylin and Eosin dyes [20,21] for the investigation of general histological changes under light microscope at magnification 400X.
Tissue Hormonal Evaluation
Preparation of tissue homogenates
Testes were removed, washed in 0.9% saline and then dried on filter paper. 100 mg tissue was homogenized in 1 ml of 1X PBS and stored overnight at -20°C. After two freeze-thaw cycles were performed to break the cell membranes, the homogenates were centrifuged for 5 min at 5000 x g, 2-8°C. The supernatant was removed and assayed immediately.
Sex hormones assay
Luteinizing hormone (LH) and follicle stimulating hormone (FSH) in MIU/mg and testosterone (T) in ng/mg were measured using enzyme linked immunosorbant assay (ELISA) kits per manufacture structure. LH levels in the testes homogenate were determined using Mouse luteinizing hormone ELISA Kit (Catalog No. CSB-E12770m), FSH levels in the testes homogenate were determined using Mouse follicle stimulating hormone ELISA Kit (Catalog No. CSB-E06871m) purchased from Cusabio Biotech Co. (CUSABIO) (Baltimore, USA). Testosterone hormone levels in the testes homogenate were determined using Mouse Testosterone ELISA Kit (Cat. No. KT-52637) purchased from Kamiya Biomedical Co.
Statistical analysis
Comparisons among groups were analyzed for statistical significance by using student t-test. Data were represented as mean ± standard error (SE). Differences were considered significant at p<0.05. Statistics were carried out using statistical analysis systems (SAS) program for comet and hormonal evaluations. Graphs and statistical analysis were executed using Sigma Plot 12 statistics software (Systat Software, Inc., San Jose, CA).
Results
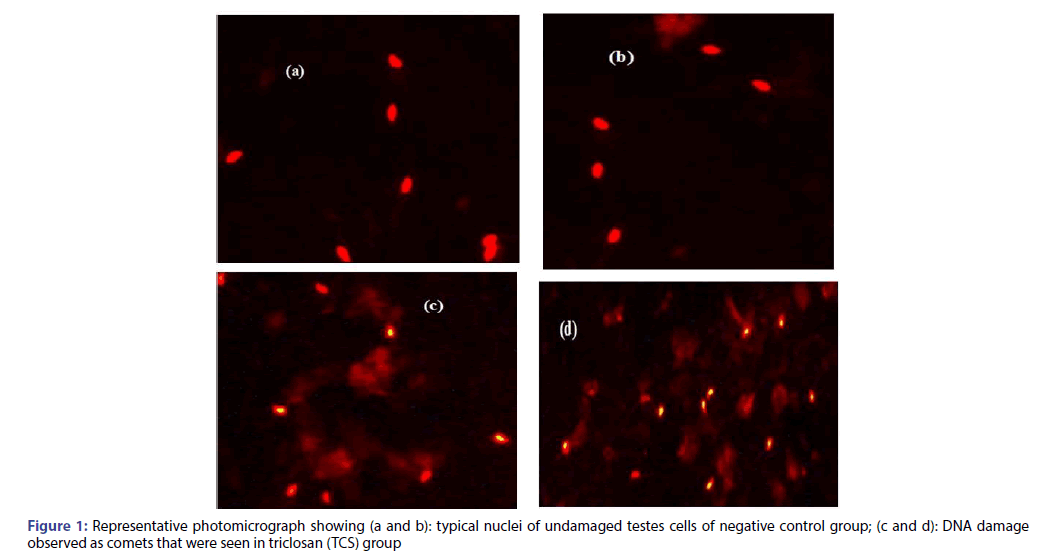
The genotoxic potential of triclosan was evaluated by using comet assay, in which Figure 1a and 1b shows typical nuclei of undamaged cells for negative control group; while Figure 1c and 1d is a representative photomicrograph for TCS group showing DNA damage observed as comets. Fifty isolated comets were randomly selected for all groups and measured for comet tail length, %DNA in tail and tail moment using COMETSCORE software.
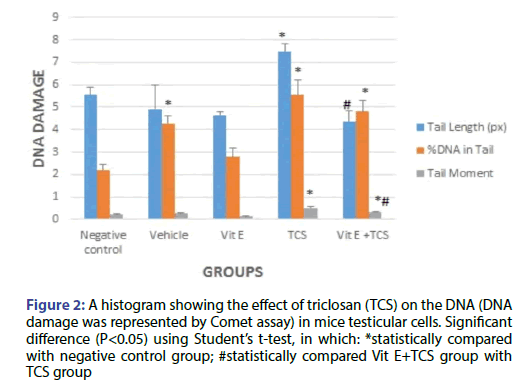
The results show a significant increase in tail length, %DNA in tail and tail moment for TCS group in comparison with the negative control group as shown in Figure 2. However, Vit E preoral administration to TCS group shows remarkable decrease in tail length but still significant in comparison to negative control group. In addition, a VitE+TCS group shows a significant reduction in %DNA in tail and tail moment in comparison to TCS group. However, for the tail moment results still significant in comparison to negative control group.
Figure 2: A histogram showing the effect of triclosan (TCS) on the DNA (DNA damage was represented by Comet assay) in mice testicular cells. Significant difference (P<0.05) using Student’s t-test, in which: *statistically compared with negative control group; #statistically compared Vit E+TCS group with TCS group
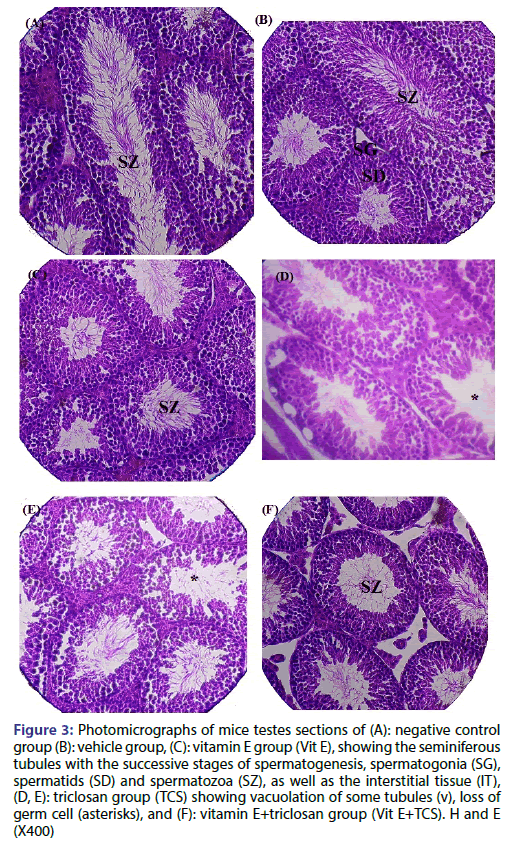
Figure 3 shows the histopathological evaluation of different testicular sections for untreated and different treated groups. In which, the testicular structure in the negative control, vehicle and Vit E mice groups show more or less normal histological architecture. The seminiferous tubules appeared uniform in size and shape; they were lined by regularly arranged rows of spermatogenic cells in different stages of maturation. Spermatogenic cells and Sertoli cells in the seminiferous tubules were observed in normal structure as shown in Figure 3a-3c respectively. In TCS group, the tubules were relatively separated from each other, the spermatocytes showed nuclear pyknosis preceding degeneration and germ cell loss. Moreover, Vacuolization and disruption of the seminiferous epithelium especially at the basal compartment present in TCS treated group indicating their location in the Sertoli cells, seminiferous epithelium hypospermatogenesis (loss of germ cells from the seminiferous epithelium) as shown in Figure 3d and 3e. Seminiferous tubule of testes sections of Vit E+TCS mice group showed testicular architecture with the regular course of spermatogenesis and mature spermatid, more or less similar to negative control group. Therefore, Vit E was able to improve spermatogenesis and reduced the loss of germ cells induced by triclosan Figure 3F.
Figure 3: Photomicrographs of mice testes sections of (A): negative control group (B): vehicle group, (C): vitamin E group (Vit E), showing the seminiferous tubules with the successive stages of spermatogenesis, spermatogonia (SG), spermatids (SD) and spermatozoa (SZ), as well as the interstitial tissue (IT), (D, E): triclosan group (TCS) showing vacuolation of some tubules (v), loss of germ cell (asterisks), and (F): vitamin E+triclosan group (Vit E+TCS). H and E (X400)
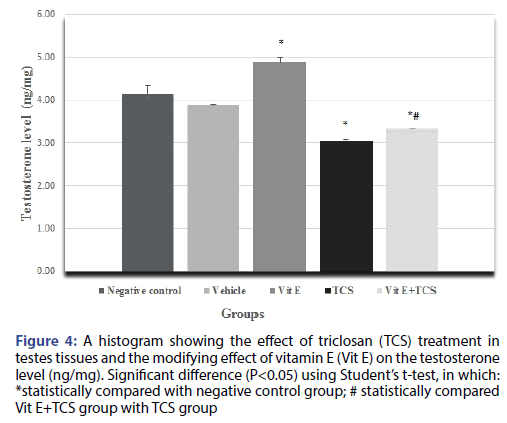
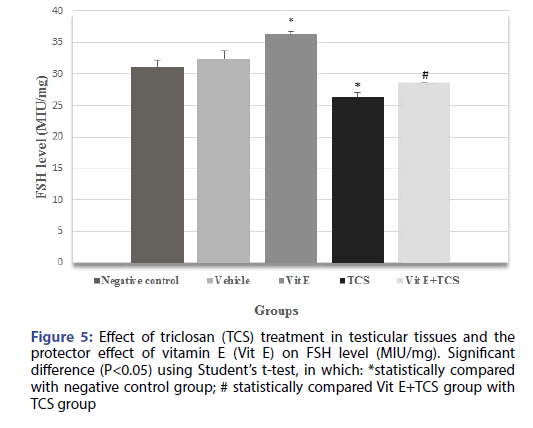
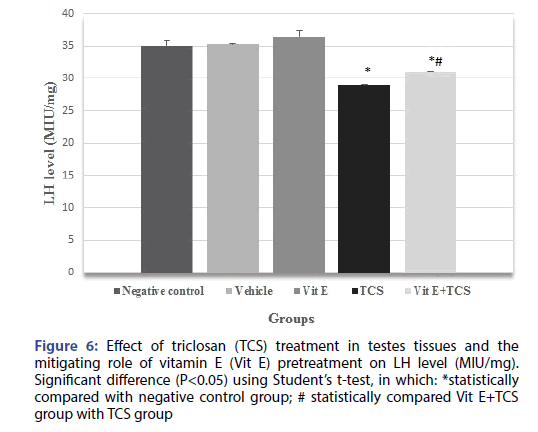
The testicular histopathological results of the TCS treated group in the current study was supported by sex hormonal dysfunction evident by significant decrease in Testosterone, FSH, LH hormones when compared with the negative control group. However, Vitamin E alleviates the effect of TCS in Vit E+TCS group by a significant increase of testosterone, FSH, LH level in comparison to TCS group as shown in Figures 4-6 respectively. Moreover, Vit E group shows a significant increase of testosterone, LH level in comparison to the negative control group as shown in Figures 4-6 respectively, and significantly enhanced the FSH level in comparison to negative control group as shown in Figure 5.
Figure 4: A histogram showing the effect of triclosan (TCS) treatment in testes tissues and the modifying effect of vitamin E (Vit E) on the testosterone level (ng/mg). Significant difference (P<0.05) using Student’s t-test, in which: *statistically compared with negative control group; # statistically compared Vit E+TCS group with TCS group
Figure 5: Effect of triclosan (TCS) treatment in testicular tissues and the protector effect of vitamin E (Vit E) on FSH level (MIU/mg). Significant difference (P<0.05) using Student’s t-test, in which: *statistically compared with negative control group; # statistically compared Vit E+TCS group with TCS group
Figure 6: Effect of triclosan (TCS) treatment in testes tissues and the mitigating role of vitamin E (Vit E) pretreatment on LH level (MIU/mg). Significant difference (P<0.05) using Student’s t-test, in which: *statistically compared with negative control group; # statistically compared Vit E+TCS group with TCS group
Discussion
The present study shows the toxic effect of TCS (15 mg/kg) treatment represented by DNA damage (represented by Comet assay), histopathological and disturbance in different testicular hormones (testosterone, FSH and LH). Our results were in agreement with previous work, in which reported [21] that treatment of male rat with higher doses of TCS (10 and 20 mg/ (kg day)) induced a significant decrease (p<0.05) in the weight of testis epididymis, ventral prostate, vas deferens and seminal vesicles and sex accessory tissues. Moreover, they assessed that in male rats treated with a dose of 20 mg/ (kg day) TCS, there was a statistically significant decrease in the serum LH (38.5%), FSH (17%), cholesterol (35%), pregnenolone (31%) and testosterone (41%) levels (p<0.05) and a number of histopathological malformations which probably affected the production and maturation of the sperms as compared to control.
Recent research demonstrates that triclosan has effects on the thyroid, estrogen, and testosterone hormones in laboratory animals, including mammals.[22-25] Moreover, TCS treated goat epididymal sperms in vitro for 5 h results in decrease of sperm motility from 77 to 46% and sperm viability from 78% to 47% in a concentration-dependent manner.[26] In addition, TCS treatment at different concentrations for different sampling time significantly decreased activities of testicular steroidogenic enzymes, 3β-HSD and 17β-HSD of goat sperms. The abnormalities observed in the testicular structures in the present study might be attributed either to the potential effect of TCS on the pituitarygonadal pathway, or to the oxidative damaging effect of free radicals.
TCS as an endocrine-disrupting chemical (EDC) in male rats has the potential to affect the pituitary-gonadal pathway at various levels because of its action at various steps of steroidogenesis: including reduction of LH and cholesterol production; depressed StAR protein expression and finally down-regulation of several key steroidogenic enzymes. That in turn impairs the androgen production and maintenance of sex accessory tissues.[21] An earlier report exists in the effect of polychlorinated biphenyls on testicular steroidogenesis through oxidative stress,[27] that might be a mechanism way for TCS as an antiandrogenic chemical.
The ROS production of TCS was later reported in few recent researches, in which [28,29] revealed that 1 and 10 m MTCS treatment on primary cultures of mouse neocortical neurons increased the production of ROS, that in turn this effect of TCS was reversed by 10 mM of the ROS scavenger, N-acetyl-L-cysteine. Moreover, it has been indirectly demonstrated that TCS contaminants increase ROS production in aquatic organisms, such as mussels or Daphnia magna.[29-31] In addition, reported that 50 mM TCS treatment directly increased the ROS formation and depleted the glutathione activity in rat neural stem cells.[32] The production of free radicals and oxidation of germ cells in the testis can reduce sperm concentration.[33,34]
Therefore, TCS (15 mg/kg) treatment for 2 consecutive days for male mice increased oxidative stress production in testicular tissue. That was cleared in the presence of DNA damage by significant increase in tail length, %DNA in tail and tail moment in comparison with the negative control group. Moreover, TCS treatment decreases different testicular hormones (testosterone, FSH and LH), that in turn affects testicular histological architecture. This hypothesis was confirmed by reversing TCS effects by the oral pre-administration of the effective ROS scavenger, Vit E.
Our results were in agreement with previous studies, they have shown that antioxidants and vitamins C, E, and B can strengthen the bloodtestis barrier, protect and repair sperm DNA, and can be effective in treating male infertility by reducing the damage caused by free radicals. [33-35] In addition, vitamin E (100 mg/kg) treatment decreased the oxidative stress and the percentage of abnormal sperms in Busulfantreated mice and increased sperm concentration and the antioxidant activity.[36-38] Moreover, vitamin E, as an antioxidant, has the ability to reconstruct seminiferous tubules after damages caused by ozone gas and reduces the harmful effects of this gas on testicular tissue and strengthens the blood-testis barrier. This research showed vitamin E to be an effective antioxidant in dealing with external and toxic factors in testicular tissue.
Conflict of Interest
The authors have declared no conflict of interest.
REFERENCES
- Darbre PD. Environmental oestrogens, cosmetics and breast cancer Res. Clin. Endocrinol. Metab 2006;20:121-43.
- Lakeram M, Lockley DJ, Sanders DJ, Pendlington R, Forbes B. Paraben transport and metabolism in the biomimetic artificial membrane permeability assay (BAMPA) and 3-day and 21-day Caco-2 cell systems. J.Biomol. Screen 2006;12:84-91.
- Cabana H, Jiwan JL, Rozenberg R, Elisashvili V, Penninckx M, Agathos SN, et al. Elimination of endocrine disrupting chemicals nonylphenol and bisphenol A and personal care product ingredient triclosan using enzyme preparation from the white rot fungus Coriolopsispolyzona. Chemosphere 2007;67:770-8.
- Heidler J, Sapkota A, Halden RU. Partitioning, persistence and accumulation indigested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ. Sci. Technol 2006;40:3634-9.
- Nakada N, Tanishima T, Shinohara H, Kiri K, Takada H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res 2006;40:3297-303.
- Black JG, Howes DRT. Percutaneous absorption and metabolism of Irgasan DP 300. Toxicol 1975;3:33-47.
- Tatarazako N, Ishibashi H, Teshima K, Kishi K, Arizono K. Effects of triclosan on various aquatic organisms. Environ. Sci 2004;11:133-40.
- Kanetoshi A, Ogawa H, Katsura E, Kaneshima H. Chlorination of IrgasanDP300 and formation of dioxins from its chlorinated derivatives. J. Chromatogr 1987;389:139-53.
- Kanetoshi A, Ogawa H, Katsura E, Kaneshima H, Miura T. Formation of polychlorinateddibenzo-p-dioxins upon combustion of commercial textile productscontaining 2,4,4-trichloro-2-hydroxydiphenyl ether (Irgasan DP300). J. Chromatogr 1998a;442:289-99.
- Kanetoshi A, Ogawa H, Katsura E, Kaneshima H, Miura T. Formation of polychlorinateddibenzo-p-dioxins from 2,4,4-trichloro-2-hydroxydiphenyl ether (Irgasan DP300) and its chlorinated derivatives by exposure to sunlight. J. Chromatogr 1998b;454:145-55.
- Foran CM, Bennett ER, Benson WH. Developmental evaluation of a potentialnon-steroidal estrogen: triclosan. Mar. Environ. Res 2000;50:153-6.
- Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, et al. Effects of triclosanonthe early life stages and reproduction ofmedaka Oryziaslatipes and induction of hepatic vitellogenin. Aquat. Toxicol 2004;67:167-79.
- Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol. Appl.Pharmacol 2007;221:278-84.
- Dayan AD. Response to: ethics, data and the pharmaceutical physician. Int. J.Clin. Pract 2006;60:1009.
- Mathur PP, Saradha B, Vaithinathan S. Impact of environmental toxicants ontesticular function. Immun. Endoc. Metab. Agents Med. Chem 2008;8:79-90.
- Yueh M, Taniguchib K, Chena S, Evansc RM, Hammockd BD, Karinb M, et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. PNAS 2014;111: 17200-5.
- Haque SE, Gilani KM. Effect of ambroxol, spirulina and Vitamin-E in naphthalene induced cataract in female rats. Indian J. Physiol. Pharmacol 2005;49:57-64.
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res 1988;175:184-91.
- Olive PL, Banath JP. Induction and rejoining of radiation-induced DNA single-strand breaks: “tail moment” as a function of position in the cell cycle. Mutat. Res 1993;294:275-83.
- Carleton HM. Carleton’s Histological Technique. Oxford University Press. 4th edition. 1967.
- Kumar V, Chakraborty A, Kural MR, Roy P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod. Toxicol 2009;27:177-85.
- Chen JG, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, et al. Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology 2008;149:1173-9.
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, et al. The Effects of Triclosan on Puberty and Thyroid Hormones in Male Wistar Rats. Toxicol Sci 2009:107:56-64.
- Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female Wistar rat. Toxicol Sci 2010;117:45-53.
- Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007-2008. Sci Total Environ 2013;445:299-305.
- Priyatha CV, Chitra KC. In vitro exposure to triclosan regulates hydroxysteroid dehydrogenase activity in epididymal sperm of goat. World J. Pharm. Sci 2016;4:69-75.
- Murugesan P, Balaganesh M, Balasubramanian K, Arunakaran J. Effects of polychlorinated biphenyl (Arochlor 1254) on steroidogenesis and antioxidant system in cultured adult rat Leydig cells. J. Endocrinol 2007;192:325-8.
- Szychowski KA, Wnuk A, Kajta M and Wójtowicz AK. Triclosan activates aryl hydrocarbon receptor (AhR)-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. Environ. Res 2016;151:106-114.
- Binelli A, Cogni D, Parolini M, Riva C, Provini A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan In Zebra mussel hemocytes. Aquat. Toxicol 2009;91:238-44.
- Riva C, Cristoni S, Binelli A. Effects of triclosan in the fresh water mussel Dreissenapolymorpha: aproteomic investigation. Aquat. Toxicol 2012;118:62-71.
- Sengupta N, Litoff EJ, Baldwin WS. The HR96 activator, atrazine, reduces sensitivity of D. magna to triclosan and DHA. Chemosphere 2015;128:299-306.
- Park BK, Gonzales ELT, Yang SM, Bang, M, Choi CS, Shin CY. Effects of triclosan on neural stem cell viability and survival. Biomol. Ther 2016;24:99-107.
- Brooks DE. Activity and androgenic control of glycolytic enzymes in the epididymis and epididymal spermatozoa of the rat. Biochem J 1976;156:527-37.
- Amin A, Hamza AA. Effects of Roselle and Ginger on cisplatin-induced reproductive toxicity in rats. Asian J. Androl 2006;8:607-12.
- Chu FF. The human glutathione peroxidase genes GPX2, GPX3, and GPX4 map to chromosomes 14, 5, and 19, respectively. Cytogenet Cell Genet 1994;66:96-8.
- Rohnavaz F, Mirzapour T, Bayrami A, Zahiri M. Antioxidant effects of alpha-tocopherol (vitamin E) on testis regeneration in busulfan-treated mice. Iran South Med. J 2016;19:511-25.
- Noumi E, Amvan ZP, Lontsi D. Aphrodisiac plants used in Cameroon. Fitotherapia 1998;69:125-34.
- Kumar R, Gautam G, Gupta NP. Drug therapy for idiopathic male infertility: rationale versus evidence. J Urol 2006;176:1307-12.