Formulation Development and Evaluation of Vancomycin HCL Nanoparticles for the Treatment of Bacterial Meningitis
2 Department of Pharmaceutics, Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology, Changa, Anand, Gujarat-388421, India
3 Department of Pharmaceutics, Coral Pharma Chem, Corel House, Sarkhej, Gota, Ahmedabad, Gujarat-382481, India
Received: 08-Sep-2021 Accepted Date: Sep 22, 2021 ; Published: 29-Sep-2021
Citation: Athalye M, Patel A. Formulation Development and Evaluation of Vancomycin HCL Nanoparticles for the Treatment of Bacterial Meningitis. J Basic Clin Pharma 2021;12(6):82-87.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
The drugs which are used for the treatment of are bacterial meningitis are ampicillin, gentamycin, cefotaxime, and vancomycin HCL, where vancomycin HCL is broad spectrum antibiotic. There are various Nano particulate drug delivery systems such as dendrimers, lipoic nanoparticles, inorganic nanoparticles and polymeric nanoparticles capable of incorporating the therapeutic drugs or imaging agents with the effective drug targeting at required site in the body. The effective treatment and diagnosis of various CNS diseases is restricted by Blood–Brain Barrier (BBB) which inhibits almost all the macromolecular drugs as well as small-molecule drugs to permeate through brain. The direct drug delivery of drugs to brain may indicate higher therapeutic activity to toxicity ratio as that of systemic drug delivery. The intranasal drug delivery is the noninvasive method through which the drug can be delivered to brain through bypassing of BBB. The Nano particulate systems indicates the enhanced drug delivery to brain as compared to the drug solution formulation through avoidance of first-pass metabolism, rapid systemic absorption, fast onset of action, increased drug bioavailability and less systemic side effects etc. Moreover, chitosan based nanoparticles are also biodegradable and stable besides their muco-adhesive character and intrinsic bioactivity. This results in increased penetration of drugs in the brain through the olfactory route and therefore they can be used for the treatment of various CNS diseases.
Keywords
Vancomycin HCL; Bacterial Meningitis; Entrapment Efficiency
Introduction
The bacterial meningitis is an inflammatory disease occurring in the protective membranes covering the brain and spinal cord and it exhibits the major symptoms such as seizures, sudden high fever, skin rash etc [1, 2]. According to WHO, one million suspected cases have been reported and 100,000 people have been died. The causative risk factors include age, definite medical conditions, working with meningitis-causing pathogens, community etc. In spite of various modern antibiotics and enhanced critical care, it is still an unsolved problem and indicates the mortality rate about 34%. Therefore, it requires the immediate antibiotic therapy and should not be delayed by the diagnostic tests. The suspected meningococcal disease patients are given antibiotic treatment but it is depends upon the medical environment and local resistance condition. For efficient antibiotic therapy, appropriate microbiological identification and susceptibility testing of the causative agent are required [3]. Chitosan as polymer has many advantages such as flexibility in surface modification biocompatibility, low immunogenicity, low toxicity and antibacterial activity. Unlike, the most of the generally used polymers, chitosan exhibits the muco-adhesive property as well is cationic in nature. This property is desirable to increase the cellular uptake of drugs via ionic interactions and to promote the drug permeation through mucous membranes [3]. Therefore, the present research work is aimed for preparation and evaluation of polymeric nanoparticles for the treatment of bacterial meningitis for nose to brain drug delivery.
Materials and Methods
Vancomycin HCL was obtained from Montage Laboratories Pvt. Ltd., Himmat Nagar, Gujarat, India as a gratis Sample. Chitosan was obtained from Sigma-Aldrich, USA and sodium tripolyphosphate, potassium dihydrogen orthophosphate, sodium dihydrogen orthophosphate, calcium chloride, sodium hydroxide pellets, sodium chloride, potassium chloride were purchased from Loba chemie, Mumbai, Maharashtra, India. All other reagents used were of analytical or equivalent grade.
Formulation development of vancomycin HCL Nano Particles (NPs)
Optimization of formulation by 32 full factorial design: The VCN-NPs were optimized by employing a 32 full factorial design with 3 factors, 2 levels and total 9 runs. The independent variables were concentration of chitosan and TPP whereas particle sizes, Poly Dispersity Index (PDI) and Entrapment Efficiency (EE) were selected as dependent variables as indicated in Table 1. The runs of the factorial design were determined through Design Expert® software (Version 10.0.3.0), the value of which are as indicated. The obtained results were analyzed statistically at 5% level of significance and best fitting model was determined. From the obtained P value, it was decided as the model terms were non significant or significant in which P value <0.05 were considered as statistically significant.
| Level | ||||
|---|---|---|---|---|
| Independent Variables | -1 | 0 | 1 | |
| X1 | Concentration of chitosan (mg) | 2 | 2.5 | 3 |
| X2 | Concentration of TPP (Tri-polyphosphate) (mg) | 1 | 2 | 3 |
| Dependent variables | ||||
| Y1 | Particle Size | |||
| Y2 | % Entrapment Efficiency | |||
| Y3 | PDI (Polydispersity Index) | |||
Table 1: List of dependent and independent variables for 32 factorial design.
Check point analysis: In the formulation of Vancomycin HCL chitosan nanoparticles, check point analysis was carried out to determine the function of contour plots and obtained polynomial equation for the prediction of the response. The two check point values of X1 and X2 (independent variables) at any one point of each contour plot were selected and then theoretical values of dependent variables were determined by substitution of the values to particular polynomial equation. Nanoparticles were formulated at 2 points experimentally and then the batches were evaluated for responses. The t-test was employed to determine the differences of the experimentally obtained mean value and theoretically computed values of dependent variables.
Optimization of formulation with desirability function: For the optimization of VCN-NPs, the desirability function was also employed to determine the level of independent variables X1 and X2 which may give minimum value of vesicle size and maximum value of EE. While performing the optimization process of formulation, to get the product of desired characteristic, the responses were combined. By combining all the responses in one experiment, the desirability function provides the possible way of prediction of optimum levels of the independent variables [4]. By considering these facts, the optimization was carried out through the software such as to get the optimized batch which indicates minimum vesicle size and maximum %EE [4].
Preparation of vancomycin HCL NPs: The Vancomycin HCL nanoparticles were formulated by Ionic gelation method in which the required quantity of chitosan was dissolved in 0.2% v/v glacial acetic acid solution (20 ml) whereas drug and sodium Tri Poly Phosphate (TPP) were added in 10 ml double distilled water. The solution of TPP was added in the solution of chitosan drop by drop at the rate of 0.5 ml/ min under constant stirring over magnetic stirrer at 800 rpm. Once the TPP solutions had been added completely, the resultant solution was kept for 100 minutes for effective cross-linking. The NPs were separated by centrifugation (Remi, India) at the speed of 10,000 rpm and at 20°C for 30 minutes. The supernant was collected to calculate Entrapment Efficiency (EE) and NPs were re-suspended. All nanoparticle batches were prepared in triplicate [5, 6].
Characterization and evaluation of optimized vancomycin HCL NPs
%Entrapment efficiency: The prepared nanoparticles were subjected to centrifugation for 30 min at 10,000 rpm into a cooling centrifuge (Remi, India). The amount of encapsulated Vancomycin HCL in the supernatant was determined at 280 nm by UV-visible spectrophotometer. The concentration of vancomycin HCL in the supernatant was determined in order to measure the free drug concentration (Cfree). This was multiplied by the volume of the supernatant to measure the total amount of drug present in the supernatant. The EE of chitosan nanoparticles was calculated according to the following equation:
%EE=Wt–Ws/Wt*100 ……………… (1)
Where EE indicates the Entrapment efficiency of nanoparticles, Wt is the total amount of Vancomycin HCL used for the formulation of nanoparticles and Ws indicates the amount of encapsulated drug in the supernatant.
Particle size and polydispersity index: VCN-NPs were subjected to centrifugation for 30 min at 10,000 rpm and supernant was obtained for performing %EE and the sediment was dispersed in 4 ml of water which was then sonicated for 6 minutes (Ultra Sonication JP-4820, India). The particle size analysis was conducted by Malvern Instruments, NanoS90, Particle size analyser. The formulated Nanoparticle formulation was diluted at least 100 times. The measurements of all the batches were conducted at 25ºC. The results were obtained in triplicate. The desired particle size for nose to brain drug delivery is less than 200 nm [7]. The higher particle size does not allow drug particle to cross nasal mucosa. Further, the polydispersity index must also be less than or equal to 0.50 as it indicates the uniformity of the particles in the formulation.
Zeta potential: The estimation of zeta potential through determination of electrophoretic mobility of the nanoparticle dispersion system was carried out by Malvern Zetasizer (S90, Malvern Zetasizer, UK). The dispersion was subjected to centrifugation for 30 min at 10,000 rpm at 10°C temperature in which supernant was rejected and the obtained resultant nanoparticles were re-suspended in double distilled water. This dispersion of nanoparticle formulation was diluted as per the requirement and then the zeta potential was determined.
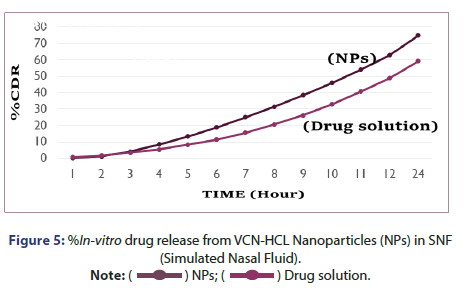
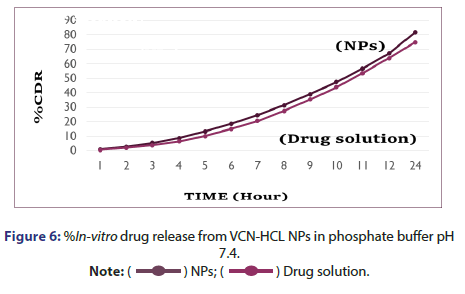
%In vitro drug release study of VCN-NPs: The %in vitro drug release study of Vancomycin HCL from NP formulation was studied using diffusion tube which is mostly used method to determine the release of drug from nanoparticles. The chitosan nanoparticles containing drug (1 ml) was kept in a dialysis tube (molecular weight cut off 12,000 Da, Himedia, India) and was immersed in a beaker containing 250 ml of pH 7.4 and Simulated Nasal Fluid (SNF). Then both the ends of the dialysis tube were clipped and the tube was suspended in a beaker. It was placed over magnetic stirrer (Remi, India) for continuous stirring at 150 rpm. The 5 ml aliquot of dissolution medium was withdrawn at various time points (1,2,3,4,5,6,7,8,9,10,11,12 and 24 hrs) and was replaced with fresh dissolution medium in the same volume. The aliquots were analysed at 280 nm using UV spectrophotometer. The concentration of drug was determined using the standard plots of the drug in phosphate buffer pH 7.4 and SNF and the %drug release was calculated at each sampling time. The results were obtained in triplicate [5, 8].
Results and Discussion
Optimization of formulation by 32 full factorial design
Evaluation of prepared nanoparticles of vancomycin HCL: The Particle size values for 9 batches indicated a greater variation which ranged from a minimum 123.3 nm to maximum 236.2 nm, for %EE ranging from a minimum 69.5% to maximum 78.21% and for PDI ranging from a minimum 0.192 to maximum 0.664. The values of all the 9 batches in the form of transformed value are as indicated in Table 2.
| Batch No. | Concentration of chitosan (mg) | Concentration of TPP (Tri-polyphosphate) (mg) | Particle Size (nm) | %EE (Entrapment Efficiency) | Polydispersity Index (PDI) |
|---|---|---|---|---|---|
| V1 | -1 | -1 | 185.2 | 69.5 | 0.452 |
| V2 | 0 | -1 | 129.1 | 74.56 | 0.481 |
| V3 | 1 | -1 | 207.9 | 77.52 | 0.664 |
| V4 | -1 | 0 | 143.7 | 74.56 | 0.302 |
| V5 | 0 | 0 | 123.3 | 72.08 | 0.277 |
| V6 | 1 | 0 | 150.8 | 76.84 | 0.381 |
| V7 | -1 | 1 | 236.2 | 77.52 | 0.192 |
| V8 | 0 | 1 | 175.6 | 78.21 | 0.267 |
| V9 | 1 | 1 | 227.3 | 77.07 | 0.205 |
Table 2 : Evaluation of prepared vancomycin HCL Nanoparticles (NPs).
For %Entrapment Efficiency
%EE=+73.24+1.64*A+1.87*B–2.12*AB+1.08*A2+1.77*B2……………… (2)
For Particle size
Particle Size=123.04+3.55*A+23.25*B+17.10*AB+29.24*A2+34.34*B2– 30.65*A2B+24.90*AB2……………………… (3)
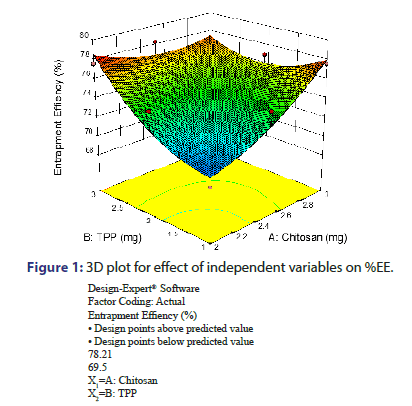
From the equation no. 2 and from the response surface 3D plot, P Value was found to be 0.0451, which is less than 0.05, shows model significant (Figure 1). TPP and Chitosan indicated positive effect on %EE as evident from the positive value of coefficient. It means that as TPP and Chitosan increases, %EE also increases. Increase in the %EE with increased chitosan concentration might be due to reason that higher amount of chitosan increases the ionic gel formation capacity. It causes increase in viscosity of the solution and hence the increased diffusional resistance of the drug molecule which prevents the vancomycin HCL movement to the external phase and thus it results in increased %EE. %EE increase with increase in TPP concentration indicates the more cross linking density of chitosan matrix in addition to participation of more chitosan molecules in the ionic gelation process. This also results in increased drug entrapment [9, 10].
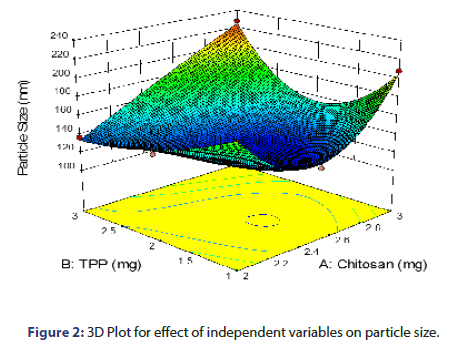
From the equation 3 and from the response surface 3D plot, P Value was found to be 0.0099, which is less than 0.05, shows model significant (Figure 2). TPP and chitosan shows positive effect on particle size as reflected by the positive value of coefficient. As the concentration of chitosan increases, particle size increases which might be due to the fact that at higher concentration of chitosan, viscosity is much higher which causes an increase in the viscous forces, resisting droplet breakdown by stirring and hence it affects the shearing capacity of stirrer and leads to increase in particle size. The increase in the particle size with the increase in the concentration of TPP could be attributed to stiffness of the cross-linking between chitosan and TPP. As the concentration of TPP increases, more tri poly phosphoric ions will be available to cross link with the amino groups on chitosan chain [9, 10].
Check point analysis: Two check point batches of formulation were formulated and evaluated for the Particle size, %EE and PDI as indicated in Table 3. Results reveal that the measured response values were as expected and at 5% level of significance, there was no significant difference between the actual and predicted value of entrapment efficiency and particle size.
| Batch code | Concentration of chitosan (mg) | Concentration of TPP (Tri-polyphosphate) (mg) | Particle size (nm) | %EE | PDI | |||
|---|---|---|---|---|---|---|---|---|
| Predicted | Actual | Predicted | Actual | Predicted | Actual | |||
| OP1 | 2.42 | 2.12 | 171.8 | 173.6 | 76.21 | 75.94 | 0.311 | 0.315 |
| OP2 | 2.35 | 2.54 | 156.2 | 154.1 | 80.87 | 80.73 | 0.237 | 0.232 |
| OP3 | 2.1 | 2.9 | 148.8 | 144.3 | 81.12 | 81.08 | 0.214 | 0.209 |
Table 3: Comparison between observed and predicted results of checkpoint batches.
Optimization of design batch of nanoparticles with desirability function: Once the effect of independent variables on the measured responses was known, then the levels of these independent variables which exhibit the optimum response were estimated. Therefore, the range of all the variables were decided and the batch which indicated a minimum value of particle size along with a maximum value of %EE and significant value of PDI was derived and selected as optimized batch. The optimised batch formula with the values of the variables is as per Table 4.
| Independent Variable | Actual Value | Desirability |
|---|---|---|
| Concentration of chitosan (mg) | 2.1 mg | |
| Concentration of TPP (Tri-polyphosphate) (mg) | 2.9 mg | 1 |
| Dependent Variable | ||
| Particle size (nm) | 144.3 | |
| %EE | 81.08 | |
| PDI | 0.209 |
Table 4: Optimised formulation as per the Design Expert® 9.0.2.0 software.
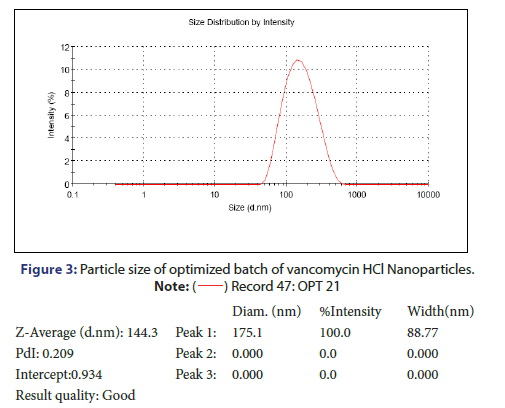
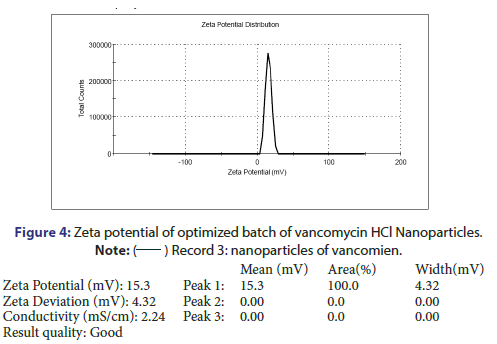
Characterization and evaluation of optimized vancomycin HCL NPs formulation: Particle size, %EE and PDI of all nanoparticle formulations were in the range of 123.3 nm to 236.2 nm, 69.5% to 78.21% and 0.192 to 0.664 respectively. The optimized batch indicated 143.3 nm Particle size, 81.08% EE and 0.209 PDI which is in the desired range (Figure 3). It is reported that nanoparticles with size <200 nm are readily transported into the brain transcellularly through olfactory neurons via endocytic pathways like sustentacular or neurons in the olfactory membrane. Poly Dispersity Index (PDI) indicates the nanoparticles distribution pattern as non-uniform particles of formulation can affect the various pharmacokinetic parameters and thus may affect the therapeutic effectiveness of the formulation also [11]. The value of zeta potential of the optimized batch was +15.3 mV as indicated in Figure 4. The positive zeta potential and greater value shows that it creates the repulsion between the nanoparticles and it prevents the aggregation of the NPs and thus results in the long-term stability [12, 13]. This higher value can be attributed to the presence of unreacted residual amino groups on the backbone of chitosan and it also signifies the optimum chitosan concentration utilized in the formulation. Moreover, the positive surface charges of chitosan nanoparticles help them to get transported through BBB via adsorptive mediated endocytosis. This is attributed to electrostatic interactions or attractions between the positively charged nanoparticles and the negatively charged endothelial surface which does not need any specific binding sites on the surfaces of the cell [11].
%In Vitro drug release from vancomycin HCL nanoparticles: In vitro drug release study was performed in two different medium in which one was pH 7.4 Phosphate buffer and another was SNF. %In vitro drug release from vancomycin HCL NPs in SNF and pH 7.4 Phosphate buffer are as indicated in Figures 5 and 6. It was observed that initially no significant amount of drug was released followed by increase in the drug release suggesting the sustained drug release pattern of formulation. This might be due to the lack of drug molecules on the surface of the polymer at the initial time points and the drug release for 24 hr suggests the slow diffusion of vancomycin HCL from the core of the nanoparticles to the surface. The slow drug release also indicates an even drug entrapment throughout the polymeric matrix [6].
Conclusion
In the present research work, the chitosan nanoparticles containing vancomycin HCL were prepared through ionic gelation method as it exhibits high stability. The formulation was prepared using Tri Poly Phosphate (TPP) as cross-linking agent based on the literature review and preliminary trial batches. On the basis of preliminary batches, two formulation variables were applied in factorial design. The 32 Full Factorial design was applied to determine the effects of 2 independent factors like Chitosan and TPP, with 3 levels for the optimization of the vancomycin HCL formulation. The particle size and Entrapment Efficiency (EE) were selected as the dependent parameters. To correlate the independent and dependent variables, factorial equations were obtained by statistical evaluation of results. Checkpoint analysis was carried out in order to validate the experimental design and desirability function was used to obtain the optimized batch. By using Design of Expert® software (version 10.0.3.0), optimized batch of NPs was selected and evaluated for various parameters like %EE, particle size, zeta potential and in vitro drug release in phosphate buffer pH 7.4 and simulated nasal fluid. The optimized batch exhibited 143.3 nm particle size, 81.08 %EE and 0.209 PDI which is in the desired range. The zeta potential of the optimized batch was +15.3 mV which indicates the long-time stability of the nanoparticles. It also indicated the release of drug in phosphate buffer pH 7.4 and simulated nasal fluid for 24 hr indicating the sustained release of drug from chitosan nanoparticles. Thus, the prepared Vancomycin HCL NPs formulation may increase the bioavailability due to increased permeation of drug through nose to brain drug delivery and due to controlled release property of nanoparticles as compared to drug solution. This may reduce the drug administration frequency, related side effects as well as may increase the patient compliance. Though, the exhaustive animal studies should be performed to establish the effectiveness of the formulation for the treatment of bacterial meningitis.
Conflict of Interest
The authors report no conflict of interest.
Acknowledgment
We are thankful to Montage Laboratories Pvt. Ltd. Himmatnagar, Gujrat, India, for providing gratis sample of Vancomycin Hydrochloride. We are also thankful to Ramanbhai Patel College of Pharmacy for providing us the facility to carry out this work.
REFERENCES
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Arch Clin Infect Dis. 2004;39(9):1267-1284.
- Hoffman O, Weber JR. Pathophysiology and treatment of bacterial meningitis. TAND. 2009;2(6):401-412.
- Manek E, Darvas F, Petroianu GA. Use of biodegradable, chitosan-based nanoparticles in the treatment of Alzheimer’s disease. mol. 2020 Jan;25(20):4866.
- Paradkar MU, Parmar M. Formulation development and evaluation of Natamycin niosomal in-situ gel for ophthalmic drug delivery. J Drug Deliv Sci Technol. 2017;39:113-122.
- Honary S, Ebrahimi P, Hadianamrei R. Optimization of particle size and encapsulation efficiency of vancomycin nanoparticles by response surface methodology. Pharm Dev Technol. 2014;19(8):987-998.
- Masjedi M, Azadi A, Heidari R, et al. Brain targeted delivery of sumatriptan succinate loaded chitosan nanoparticles: Preparation, In vitro characterization, and (Neuro-) pharmacokinetic evaluations. J Drug Deliv Sci Technol.2021.
- Fazil M, Md S, Haque S, et al. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci. 2012;47(1):6-15.
- Ong WY, Shalini SM, Costantino L. Nose-to-brain drug delivery by nanoparticles in the treatment of neurological disorders. Curr Med Chem. 2014;21(37):4247-4256.
- Papadimitriou S, Bikiaris D, Avgoustakis K, et al. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr Polym. 2008;73(1):44-54.
- Pagar K, Vavia P. Rivastigmine-loaded L-lactide-depsipeptide polymeric nanoparticles: Decisive formulation variable optimization. Sci Pharm. 2013;81(3):865-888.
- Wilson B, Alobaid BN, Geetha KM, et al. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer's disease. J Drug Deliv Sci Technol. 2021;61:102176.
- Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1):146-157.
- Wang X, Chi N, Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm. 2008;70(3):735-740.