Thrombolytic potential of Ocimum sanctum L., Curcuma longa L., Azadirachta indica L. and Anacardium occidentale L.
- *Corresponding Author:
- Md. Razibul Habib
Department of Pharmacy, East West University, Dhaka, Bangladesh
E-mail: mrhjewel@gmail.com
Date of Received: 13-06-2011
Date of Accepted: 23-06-2011
Available Online: 15-08-2011
Abstract
Atherothrombotic diseases such as myocardial or cerebral infarction are serious consequences of the thrombus formed in blood vessels. Thrombolytic agents are used to dissolve the already formed clots in the blood vessels; however, these drugs have certain limitations which cause serious and sometimes fatal consequences. Herbal preparations have been used since ancient times for the treatment of several diseases. The aim of this study was to investigate whether herbal preparations possess thrombolytic activity or not. An in vitro thrombolytic model was used to check the clot lysis ef-fect of four aqueous herbal extracts viz., O. sanctum, C. longa, A. indica, A. occidentale along with Streptokinase as a positive control and water as a negative control. The percentage (%) clot lysis was statistically significant (p<0.0001) when compared with vehicle control. Using an in vitro thrombolytic model, O. sanctum, C. longa, A. indica & A. occidentale showed moderate clot lysis activity (30.01 ± 6.168%, 32.94 ± 3.663%, 27.47 ± 6.943%, 33.79 ± 2.926% respectively) whereas standard streptokinase showed 86.2 ± 10.7 % clot lysis effect. From our study we found that all the herbs showed reasonable % of clot lysis. These herbal extracts possess thrombolytic properties that could lyse blood clots in vitro; however, in vivo clot dissolving properties and active component(s) of these extracts for clot lysis are yet to be discovered.
Keywords
O. sanctum, C. longa, A. indica, A. occidentale, thrombolysis
Introduction
Thromboembolic disorders such as pulmonary emboli, deep vein thrombosis, strokes and heart attacks are the main causes of morbidity and mortality in developed countries. Thrombolytic therapy uses drugs called thrombolytic agents, such as alteplase, anistreplase, streptokinase, urokinase, and tissue plasminogen activator (TPA) to dissolve clots. Thrombolytic therapy is also used to dissolve blood clots that form in catheters or tubes put into people’s bodies for medical treatments, such as dialysis or chemotherapy. However, the relatively weak substrate specificity of fi rst generation agents (streptokinase and urokinase) can result in a state of systemic fi brinolysis and associated bleeding complications. Because of the shortcomings of the available thrombolytic drugs, attempts are underway to develop improved recombinant variants of these drugs [1-5]. Recently, preventive measures against thrombosis have been tried. Oral administration of the fi brinolytic enzyme nattokinase was one example, which has been reported to enhance fi brinolytic activity in plasma and the production of tPA [6].
Since ancient times, herbal preparations have been used for the treatment of several diseases. The leaves and/or twigs, stem, bark and underground parts of plants are most often used for traditional medicines. Herbal products are often perceived as safe because they are “natural” [7]. Considerable efforts have been directed towards the discovery and development of natural products from various plant and animal sources which have antiplatelet [8,9], anticoagulant [10,11], antithrombotic [12], and thrombolytic activity. Epidemiologic studies have provided evidence that foods with experimentally proved antithrombotic effect could reduce risk of thrombosis. Herbs showing thrombolytic activity have been studied and some significant observations have been reported [13]. Ocimum sanctum belongs to Lamiaceae family and is known by a common name of “Tulsi” in India & Bangladesh. Fixed oil of Ocimum sanctum increases blood clotting [14] time and percentage increase was comparable to aspirin and could be due to inhibition of platelet aggregation. Turmeric (Curcuma longa) inhibits platelet aggregation [15].
The aim of our work was to investigate whether our selected herbal plants (aqueous extract of Ocimum sanctum, Curcuma longa, Azadirachta indica & Anacardium occidental) possess thrombolytic activity or not by using an in-vitro procedure.
Materials and Methods
Streptokinase (SK)
To the commercially available lyophilized SK vial (Polamin Werk GmbH, Herdecke, Germany) of 15, 00,000 I.U., 5 ml sterile distilled water was added and mixed properly. Th is suspension was used as a stock from which 100 μl (30,000 I.U) was used for in vitro thrombolysis [16].
Specimen
Whole blood (5 ml) was drawn from healthy human volunteers (n = 10) without a history of oral contraceptive or anticoagulant therapy (using a protocol approved by the Institutional Ethics Committee of Central India Institute of Medical Sciences, Nagpur). 500 μl of blood was transferred to each of the ten previously weighed alpine tubes to form clots.
Collection and extraction
Different parts of Ocimum sanctum (leaves), Curcuma longa (Rhizomes), Azadirachta indica (leaves) & Anacardium occidentale (Fruits/Nuts) was collected at their fully mature form, from Chittagong, Bangladesh. The plant parts were identified by Bangladesh forest research institute (BFRI), Chittagong. After cleaning, the plant parts of selected plant were taken and air dried for 10 days, and then kept in an oven at 45°C at 72 hours. Then the dried plant parts were grinded. After grinding the glass extractor was used for extraction process. 40gm of dried powder was taken in the glass extractor. Before placing, the extractor was washed properly and then dried. Then 500ml of solvent methanol was added gradually & extraction was done.
Herbal Preparation
100 mg extract was suspended in 10 ml distilled water and the suspension was shaken vigorously on a vortex mixer. The suspension was kept overnight and decanted to remove the soluble supernatant, which was fi ltered through a 0.22 micron syringe fi lter. 100 μl of this aqueous preparation of herbs was added to the alpine tube containing the clots to check thrombolytic activity [16].
Clotlysis
Experiments for clotlysis were carried as reported earlier [16]. Venous blood was drawn from healthy volunteers (n = 10) and transferred in different pre-weighed sterile alpine tube (500 μl/tube) and incubated at 37°C for 45 minutes. After clot formation, serum was completely removed (aspirated out without disturbing the clot formed). Each tube having clot was again weighed to determine the clot weight (Clot weight = weight of clot containing tube – weight of tube alone). Each alpine tube containing clot was properly labeled and 100 μl of plant extract was added to the tubes. As a positive control, 100 μl of SK and as a negative non thrombolytic control, 100 μl of distilled water were separately added to the control tubes numbered. All the tubes were then incubated at 37°C for 90 minutes and observed for clotlysis. After incubation, fluid obtained was removed and tubes were again weighed to observe the difference in weight after clot disruption. Difference obtained in weight taken before and after clotlysis was expressed as percentage of clotlysis. The test was repeated ten times.
Statistical analysis
The significance of % clotlysis by herbal extracts by means of weight difference was tested by the paired t-test analysis. Data are expressed as mean ± standard deviation.
Results
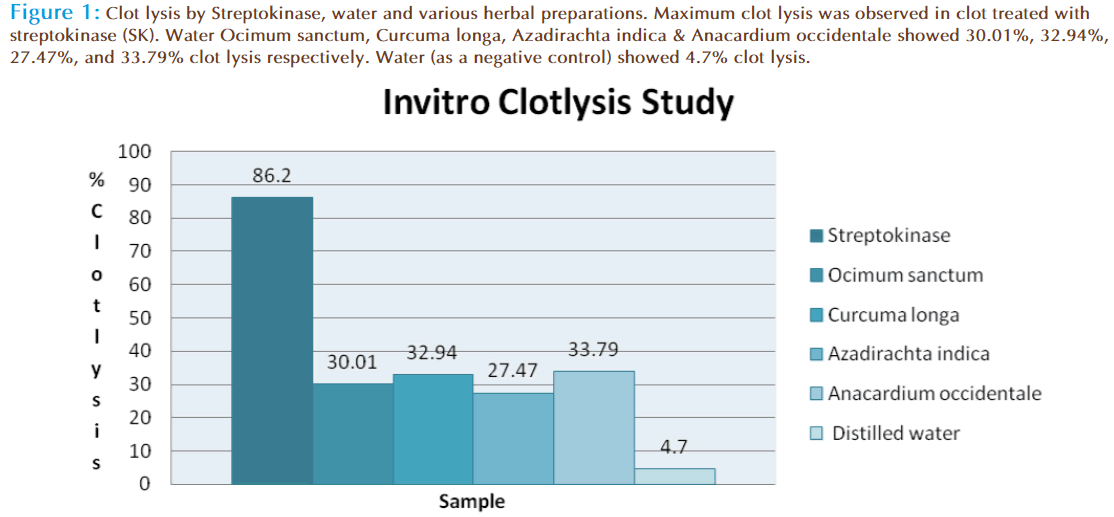
Addition of 100 μl SK, a positive control (30,000 I.U.) to the clots along with 90 minutes of incubation at 37°C, showed 86.2% clotlysis. Clots when treated with 100 μl sterile distilled water (negative control) showed only negligible clotlysis (4.7%). The mean difference in clotlysis percentage between positive and negative control was very significant (p value < 0.0009). After treatment of clots with 100 μl of Ocimum sanctum, Curcuma longa, Azadirachta indica & Anacardium occidentale moderate clotlysis i.e., 30.01%, 32.94%, 27.47%, and 33.79% respectively was obtained and when compared with the negative control (water) the mean clotlysis % difference was significant (p value < 0.0001). Percent clotlysis obtained after treating clots with different herbs and appropriate controls is shown in Figure 1. Statistical representation of the effective clotlysis percentage by four herbal preparations, positive thrombolytic control (Streptokinase) and negative control (sterile distilled water) is tabulated in Table 1.
| Herb/Drug | % Clotlysis (mean ± S.D) | P value (Two-tailed) when compared to negative control (water) |
|---|---|---|
| Streptokinase | 86.2 ± 10.7 | < 0.0009 |
| Ocimum sanctum | 30.01 ± 6.168 | < 0.0001 |
| Curcuma longa | 32.94 ± 3.663 | < 0.0001 |
| Azadirachta indica | 27.47 ± 6.943 | < 0.0001 |
| Anacardium occidentale | 33.79% ± 2.926 | < 0.0001 |
Statistical representation of the effective clotlysis percentage by herbal preparations, positive thrombolytic control (Streptokinase) and negative control (sterile distilled water) done by paired t-test analysis; clotlysis % is represented as mean ± S.D. and p values of all Herbal preparations were < 0.05 was considered as significant.
Table 1: Effect of herbal extracts on in vitro clotlysis.
Figure 1: Clotlysis by Streptokinase, water and various herbal preparations. Maximum clotlysis was observed in clot treated with streptokinase (SK). Water Ocimum sanctum, Curcuma longa, Azadirachta indica & Anacardium occidentale showed 30.01%, 32.94%, 27.47%, and 33.79% clotlysis respectively. Water (as a negative control) showed 4.7% clotlysis.
Discussion
Now-a-days, about 30% of the pharmaceuticals are prepared from plants worldwide [17]. A number of studies have been conducted by various researchers to fin'd out the herbs and natural food sources and their supplements having antithrombotic (anticoagulant and antiplatelet) effect and there is evidence that consuming such food leads to prevention of coronary events and stroke [18-21]. There are several thrombolytic drugs obtained from various sources. Some are modified further with the use of recombinant technology in order to make these thrombolytic drugs more site specific and effective [20]. Side effects related to these drugs have been reported thatlead to further complications [21]. Sometimes the patients die due to bleeding and embolism [22,24-26].
In our study to evaluate thrombolytic properties of different plant extracts, we have tried Ocimum sanctum, Curcuma longa, Azadirachta indica & Anacardium occidentale preparations. These are used since ancient times for curing vascular diseases & many other diseases. For example, Fagonia arabica (Dhamasa) was reported to have antithrombotic activity [16]. There are few more plant extracts/ products which have been identified to have fi brinolytic activity. These are Lumbricus rubellus [26], Pleurotus ostreatus [29], Spirodela polyrhiza [30], Flammulina velutipes [31], and Ganoderma lucidum [32], Ginger (Zingiber officinale) [33], Garlic (Allium sativum) [34].
In this study, Streptokinase (SK), a known thrombolytic drug is used as a positive control [27]. Water, on the other hand, was selected as a negative control. The comparison of positive control with negative control clearly demonstrated that clot dissolution does not occur when water was added to the clot. By comparing with this positive & negative control, a significant thrombolytic activity was observed after treating the clots with Ocimum sanctum, Curcuma longa, Azadirachta indica & Anacardium occidentale extracts with P value (Two-tailed) less than 0.0001.
Conclusion
From this experiment, it can be concluded that the extracts of Ocimum sanctum, Curcuma longa, Azadirachta indica & Anacardium occidentale showed moderate to good clotlysis activity. Once found these herbal preparations may be incorporated as a thrombolytic agent for the improvement of the patients suffering from Atherothrombotic diseases. Th is is only a preliminary study and to make fin'al comment the extract should thoroughly investigated phytochemically and pharmacologically to exploit their medicinal and pharmaceutical potentialities.
Conflict of Interest
There are no conflicts of interest.
References
- Nicolini FA, Nichols WW, Mehta JL, Saldeen TG, Schofield R, Ross M, Player DW, Pohl GB, Mattsson C. Sustained reflow in dogs with coronary thrombosis with K2P, a novel mutant of tissue plasminogen activator. J Am Coll Cardiol. 1992; 20:228-235.
- Adams DS, Griffin LA, Nachajko WR, Reddy VB, WeicM. A synthetic DNA encoding a modified human urokinase resistant to inhibition by serum plasminogen activator inhibitor. J Biol Chem. 1991; 266:8476-8482.
- Lijnen HR, Vanhoef B, DeCock F, Okada K, Ueshima S, Matsuo O. On the mechanism of fi brin-specific plasminogen activation by staphylokinase. J Biol Chem. 1991; 266:11826-32.
- Marder VJ. Recombinant streptokinase – opportunity for an improved agent. Blood Coagul Fibrinolysis. 1993; 4: 1039-1040.
- Wu DH, Shi GY, Chuang WJ, Hsu JM, Young KC, Chang CW. Coiled coil region of streptokinase gamma-domain is essential for plasminogen activation. J Biol Chem 2001; 276: 15025-33.
- Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of fi brinolytic activity in plasma by oral administration of nattokinase. Acta Haematol 1990;84(3):139-43.
- Gesler WM. Therapeutic landscapes: medical issues in light of the new cultural geography. Soc Sci Med 1992; 34:735-46.
- Demrow HS, Slane PR, Folts JD. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation 1995; 91: 1182-88.
- Briggs WH, Folts JD, Osman HE. Administration of raw onion inhibits platelet- mediated thrombosis in dogs. J Nutr 2001;131:2619-22.
- Leta GC, Mourão PA, Tovar AM. Human venous and arterial glycosaminoglycans have similar affinity for plasma low-density lipoproteins. Biochim Biophys Acta 2002; 1586: 243-253.
- Zhiguang L, Hongli W, Jiazeng L, Zhang G, Gao C. Basic and clinical study on the antithrombotic mechanism of glycosaaminoglycan extracted from sea cucumber. Chin Med J 2000;113:706-711.
- Rajapakse N, Jung WK, Mendis E, Moon SH, Kim SK. A novel anticoagulant purified from fi sh protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sciences 2005;76: 2607-2619.
- Yamamoto J, Yamada K, Naemura A, Yamashita T, Arai R. Testing various herbs for antithrombotic effect. Nutrition 2005;21:580-587.
- Singh S, Rehan HM, Majumdar DK. Effect of Ocimum sanctum fi xed oil on blood pressure, blood clotting time and pentobarbitone-induced sleeping time. J Ethnopharmacol 2001;78:139-43.
- Srivastava R, Puri V, Srimal RC, Dhawan BN. Effect of curcumin on platelet aggregation and vascular prostacyclin synthesis. Arzneimittelforschung 1986;36:715-17.
- Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Effect of Fagonia arabica (Dhamasa) on in vitro thrombolysis. BMC Complementary and Alternative Medicine 2007;7:36.
- Anwar AK, Ashfaq M, Nasveen MA. Pharmacognostic studies of selected indigenous plants of Pakistan. Pakistan Forest Institute, Peshawar NWFP, Pakistan; 1979;15-35.
- Gillman MW, Cupples LA, Gagnon D, Posner BM, Ellison RC, Castelli WP, Wolf PA. Protective effect of fruits and vegetables on development of stroke in men. JAMA 1995;273:1113-1117.
- Joshipura KJ, Ascherio A, Manson JE, Stampher MJ, Rimm EB, Speizer FE. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999;282:1233-39.
- Liu S, Manson JE, Lee I-M, Cole SR, Hennekens CH, Willett WC, Buring JE. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr 2000;72:922-28.
- Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the fi rst National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr 2002;76:93-99.
- Verstraete M. Third generation thrombolytic drugs. Am J Med 2000;109:52-58.
- Baruah DB, Dash RN, Chaudhari MR, Kadam SS. Plasminogen activators: A comparison. Vascular pharmacology 2006;44:1-9.
- Gallus AS. Thrombolytic therapy for venous thrombosis & pulmonary embolism. Bailliere’s Clinical Haematology 1998;11:663-73.
- Wardlaw JM, Berge E, del Zoppo G, Yamaguchi T. Thrombolysis for acute ischemic stroke. Stroke 2004;35:2914-15.
- Capstick T, Henry MT. Efficacy of thrombolytic agents in the treatment of PE. Eur Respir J 2005;26:864-74.
- Tillet WS, Garner RL. The fi brinolytic activity of hemolytic streptococci. J Exp Med 1933;58:485-502.
- Jeon OH, Moon WJ, Kim DS. An anticoagulant/fi brinolytic protease from Lumbricus rubellus. J Biochem Molec Biol 1995;28:138-42.
- Choi HS, Shin HH. Purification and characterization of a fi brinolytic protease in Pleurotus ostreatus. Mycologia 1998;90:674-79.
- Choi HS, Sa YS. Fibrinolytic and antithrombotic protease from Spirodela polyrhiza. Biosci Biotechnol Biochem. 2001;65:781-86.
- Shin HH, Choi HS. Purification and partial characterization of a metalloprotease in Flammulina velutipes. J Microbiol 1998;36:20-25.
- Choi HS, Sa YS. Fibrinolytic and antithrombotic protease from Ganoderma lucidum. Mycologia 2000;92:545-52.
- Verma SK, Bordia A. Ginger, fat and fi brinolysis. Indian J Med Sci 2001;55:- 83-86.
- Bordia A, Verma SK, Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fi brinogen and fi brinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids 1998;58:257-63