Therapeutic drug monitoring by reverse Iontophoresis
- *Corresponding Author:
- Anroop B Nair
M.M. College of Pharmacy, MM University, Mullana, Ambala, India-133207
E-mail: banroop@gmail.com
Date of Received : 20-10-2011
Date of Accepted : 12-12-2011
Available online : 15-02-2012
Abstract
Therapeutic molecules possessing distinct pharmacokinetic variation, narrow therapeutic index and concentration dependent therapeutic/adverse effects demand constant monitoring. The current methods for blood sampling are invasive and possess low patient compliance. Human skin, selective and effective membrane to chemical permeation, offers an alternative route for the extraction of endogenous molecules in the body. Significant attention has been received in the application of reverse iontophoresis in extracting drugs/biomaterials from the subdermal region. This technique involves transiting of a low electric current across the skin usually with couple of skin electrodes to extract charged as well as neutral molecules. Electromigration and electroosmosis are the two basic mechanisms involved in transport of molecules. Several in vitro and in vivo experiments demonstrated the potential of reverse iontophoresis as a noninvasive tool in clinical chemistry and therapeutic drug monitoring. This technology is currently being used in device such as Glucowatch Biogrpaher which allows blood glucose detection across skin layers. Advances in technology and rapid progress in research has widely improved the opportunity of this system, and the recent trend indicates that several products are likely to be developed very soon. This review provides an overview about the recent developments in reverse iontophoresis for therapeutic drug monitoring.
Keywords
Reverse iontophoresis, Therapeutic drug monitoring, Electromigration, Electroosmosis, and Glucowatch.
Introduction
Therapeutic drug monitoring (TDM) was introduced in the early 1970s with the objectives of improving the patient safety, individualize the dosage regimen and surmount the systemic toxicity. In general, clinical tests are performed very frequently to determine the drug concentration in the body during the therapy. Essentially, TDM is highly required for drugs possessing narrow therapeutic index as a slight variation in the therapeutic range could resultin no or low clinical efficiency or causes significant side effects or high risk of toxicity [1]. However, the existing methods for drug collection such as blood sampling fromvein (venipuncture) and finger stick method is highly invasive and possess low patient compliance especially when the sampling frequency is much high .
Recently, research has been focused on non-invasive methods to monitor the drug plasma concentration. In this contest, skin provides a unique gateway for noninvasive transdermal drug monitoring. The application of electric current across the skin could be used to extract the endogenous molecules within or beneath the skin [2]. In recent days, reverse iontophoresis technique has been attempted for the non invasive drug monitoring. Typically, it applies a low electric current through a pair of skin electrodes to promote the transport of both charged and neutral molecules [3]. Several in vitro and in vivo studies have demonstrated the potential of reverse iontophoresis to monitor the sub dermal concentration of drugs and other biomolecules [4-10]. The development of biosensors have replaced the old technology of drug analysis from the extraction chamber and made novel devices which are portable and more convenient. One of the most successful applications of reverse iontophoresis till today is the noninvasive glucose monitoring employing the Glucowatch. Th is article discusses the basic principles and the recent developments on reverse iontophoresis in therapeutic drug monitoring.
Therapeutic Drug Monitoring
Maintaining the therapeutic concentration is not easy as several factors such as ADME could influence or alter the systemic drug availability. Further the monitoring is very necessary for vulnerable patient populations (neonates and geriatric) wherein a slight variation of drug concentration can likely to causes immediate adverse effects. It helps in the individualization of dosage by maintaining plasma blood concentration and thereby avoidsvariation in the pharmacokinetic and pharmacodynamic activities. Typically, TDM involves the measurement and interpreting drug concentrations in biological fluids (usually serum) and applying well-described pharmacokinetic and pharmacodynamic principles of the drug to optimize a treatment regimen for an individual patient. Since the initiation of this service, different studies evaluating TDM laboratory activities, studying the impact of the service on patient outcome, surrogate endpoints, and pharmacoeconomic evaluations have been done worldwide [11]. Therapeutic drug monitoring is very essential for the drugs which exhibit specific characteristics such as narrow therapeutic index, concentration related therapeutic and adverse effects, noticeable pharmacokinetic variation, distinct therapeutic concentration range, desired therapeutic effect difficult to monitor. TDM is considered to be most useful if there is a good correlation between the concentration of the drug and its therapeutic effect. In pediatric patients TDM establishes a reference for the treatment of epilepsy. Determination of carbamazepine concentrations in children helps in avoiding toxicity. Further, the determination of phenytoin concentration in older patients plays a key role. TDM is performed due to the reason that certain drugs have short therapeutic index; the concentration above the range causes toxicity and below the range causes ineffectiveness [12].There are situations wherein it is difficult in differentiating the disease condition and side effects of drug. In these conditions, TDM provides adequate information to decide the dosage regimen. There are also other cases in which TDM plays a major role, some of them are as follows.
• Patients having liver and renal disease
• Patients suffering from hypoalb
• Patients taking chemotherapy of cancer
• In case of infants
• Changing the dose or formulation
• Changing concomitant medication
Most of the drugs which are therapeutically monitored are taken for lifetime [11]. The steady state concentrations of these drugs must be maintained as there is increase in age of patients and also conditions like pregnancy, infections, accidents and diseased state affects the drug interaction in the body. It is likely that the TDM could provide the variation in drug concentration in the body and could help in modify the dosage regimen. Further, the noncompliance of the patients can be identified by TDM and can tailor the dosages to suit the patients. There are different classes of drugs which are monitored routinely and are listed in Table 1.
| Categories | Drugs |
|---|---|
| Analgesics | Acetaminophen, Salicylate |
| Antianginal | Perhexiline |
| Antiarrhythmic | Amiodarone, Digitoxin, Digoxin, Disopyramide, Flecainide, Lignocaine, Mexilitine, Procainamide, Propranolol, Quinidine. |
| Antibiotics | Amikacin, Choramphenicol, Gentamicin, Kanamycin, Netilmycin, Tobramycin, Vancomycin |
| Antidepressants | Lithium and Tricyclic depressants (Amitriptyline, Desipramine, Doxepin, Imipramine, Nortiptyline) |
| Antiepileptic | Carbamazepine, Ethosuximide, Gabapentin, Lamotrigine, Phenobarbital, Phenytoin, Primidone, Valproic acid. |
| Antineoplastics | Methotrexate |
| Bronchodilators | Aminophylline, Caffeine,Theophylline. |
| Immunosuppresents | Azathioprine, Cyclosporine, Mycopheno latemofetil, Sirolimus Tacrolimus |
| Protease inhibitors | Atazanavir, Indinavir, Lopinavir, Nelfinavir, Ritonavir, Saquinavir. |
Table 1: Drug molecules that necessitate therapeutic drug monitoring.
Extraction of Drugs
Skin is the largest organ of the body with an area of approximately 2m2, and provides a comprehensive and accessible surface for drug extraction from the subdermal region. The stratum corneum, outermost layer of skin, composed of dead, flat skin cells and is mainly responsible for its barrier function. Several physical and chemical approaches have been developed to compromise this formidable barrier although very few could find their way [13, 14]. Among the methods developed, iontophoresis is found to be most promising approach and has been widely studied. Iontophoresis evolved as a transdermal enhancement technique in the 20th century, primarily for the delivery of large and charged molecules [15]. Significant achievements have been made in the understanding of underlying mechanisms of iontophoresis and these have contributed to the rational development of iontophoretic delivery systems. Basically, transdermal iontophoresis apply a small electrical current (0.1-0.5 mA/cm2) to the skin in order to increase the percutaneous molecular transport. Th is technique has found its applications in the field of transdermal drug delivery and several iontophoretic drug delivery systems have reached the market.
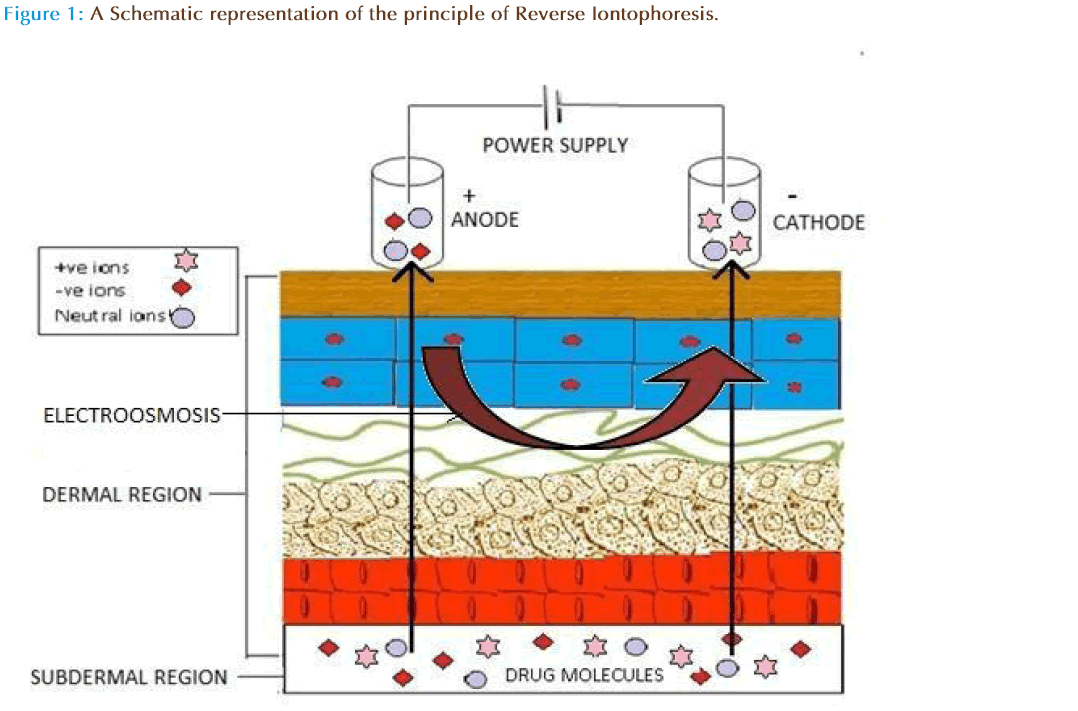
The success of transdermal iontophoresis leads the drug delivery scientists to focus on the feasibility of iontophoretic extraction (reverse iontophoresis) of molecules from the body rather than its forward iontophoreticdelivery into the body. Further, the symmetry of iontophoresis is such that it is possible to deliver molecules into the body and could also be extracted. In principle, reverse iontophoresis causes extraction of solute molecules from sub dermal parts to skin surface [16]. The technical description of this process is a non-invasive method of expelling charged drug across the skin by repulsive electromotive force using a small electrical charge applied to an iontophoretic chambers containing vehicle. Drug molecules (charged/uncharged) will be transported from the subdermal region to the electrode by different routes (intracellular, hair follicles and sweat ducts).
Drug extraction from the interstitial fluid can be achieved by transiting of a low level of a current across the skin usually with couple of skin electrodes involving a conducting base to elevate extraction of charged as well as neutral molecules, by different mechanisms. The interstitial fluid is collected and analyzed using a suitable device.Th is technique can have important applications in medical diagnostics as it can allow non-invasive sampling of endogenous molecules. In reverse iontophoresis, the negative charge of the skin at buffered pH causes it to be permselective to cations causing solvent flow towards the cathode [17]. Th is flow is the dominant force allowing movement of neutral molecules, including glucose, across the skin. The redox reactions take place at site of electrodes causes charge imbalance at both cathodic and anodic solutions as a resultion migrate to skin to maintain electro neutrality [16].Th is technique is currently being used in device such as Glucowatch which allows blood glucose detection across skin layers using reverse iontophoresis. However, ions possessing specific charges could be easily extracted by electro-repulsion [18]. Moreover, it is required that the concentration of compounds extracted from the body is directly related to its sub dermal concentration [19]. The major factor which needs to be considered during iontophoresis is the operating current density. In general, the current density of ≤0.5mA/cm2is well tolerated in humans. In experimental situations, aqueous solutions/ buffers are used to fill electrolyte chambers in which analyte is extracted, while for commercial purposes, the use of gel or polymer is preferred [15, 20]. In addition, the favorable physiochemical properties of a molecule could augment the reverse iontophoretic extraction. Charge, molecular weight, and lipophilicity are critical, and promising results can be expected for small, mobile and hydrophilic drugs. Moreover, it is important to realize that the concentration of the analyte is the variable of interest and will depend on the dosage regimen and the relevant pharmacokinetics.
The major challenge of monitoring blood levels of endogenous molecules is the possible contamination from significant analyte amounts in the skin. The sensitivity of the analytical procedures determines the minimum length of the iontophoretic sampling period that extracts sufficient amount of the analyte to allow accurate quantification. Moreover, one should remember that the drug is not extracted from the blood but it is from the skin tissues, thus represents the subdermal drug concentration. Iontophoresis fluxes of extraction are proportional to the analyte concentration in the subdermal interstitial fluid and their information about plasma levels will depend on the rapidity with which the equilibrium plasma interstitial fluid levels are established. Hence, requirement of sufficient analyte in the subdermal concentrations is essential for the successful transport and extraction of measurable amount of drug transport across the skin during reverse iontophoresis. Th is is not only challenging in the case of endogenous molecules, but also for the monitoring of drugs, especially if they are protein bound [21].Th is was demonstrated in the case of highly protein bind drug such as valproic acid and phenytoin. The authors observed that the addition of protein (albumin) to the subdermal solution reduced the drug extraction [20, 21]. Hence, it is likely that that only the free drug will be transported across the skin as the protein bound form is too large to be extracted.
A successful application of reverse iontophoresis in therapeutic drug monitoring clinically has so far been demonstrated for lithium, a small, non protein bound positively charged ion. It possesses high mobility and competes to transport charge across the skin much more efficiently than most therapeutic drugs. Moreover, the plasma concentration of lithium is also much high. Accordingly, the amounts detected at the skin surface can be easily assayed with existing technology.
Issues with reverse iontophoresis in drug monitoring continued with related to multiple aspects. The foremost issues including the requirement of complicated technology, relatively long warm-up time, and calibration with ablood sample etc. Secondly, issues related to skin (erythema, edema), incorrect observations during sweating and high cost has also limited the success of this technology. However, the snags of this technology have been rectified to some extent. The major issues such as invasive calibration have been avoided by doing the simultaneous extraction of a known internal standard [22]. The basic principle involved here is the subdermalconcentration of the analyte of interest can bepredicted solely from ratio of extracted fluxes of theanalyte and internal standard. Th is has been demonstrated in various studies using different analytes including amikacin, valproic acid, lactate, phenytoin and lithium [20-21, 23-26]. However, the challenges still remain with neutral molecules such as glucose. On the other hand, the skin may also accumulate the drug and produces a specific drug reservoir, which might be extracted in the initial stage of the reverse iontophoresis and may leads to false results. The possible option to surmount this issue could be excluding the data in the initial period, during which the drug in the skin tissues will be extracted.
Transport Mechanisms
Several reviews on the mechanisms of transport underlying iontophoresis and its applications for drug delivery are available elsewhere [15]. Iontophoresis is considered as a symmetric process, which means that molecules within the subdermal compartment can be extracted to the skin surface. The mechanism of reverse iontophoresis is as similar to iontophoresis, based on the principles of charges (i.e., like charges repel each other). An electrode with a defined charge is used to repel a drug with a similar charge, which is attracted by an oppositely charged electrode placed elsewhere on the body. Briefly, both electromigration and electroosmosis contribute to iontophoretic transport, allowing sampling of both charged and neutral molecules. The increased flux during iontophoresis would include, flux due to the electrochemical potential gradient (electromigration) across the skin and electro-osmotic water flow (resulting from solvent drag).
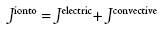
where Jelectric is the flux due to electric current application and Jconvective is the flux due to convective transport due to electroosmosis.
In electromigration, there is direct encounter between applied field and charged ion [27-29]. The power source maintains an electric field that causes migration of electrons in the electrical compartment of skin and ion starts to flow in the ionic parts. According to the total number of electrons passing the electrical compartment is totally balanced by the quantity of charge passing through the body [30]. Ionic transport maintains electro-neutrality by passing through the body [31, 32].
Faraday’s law can be used to relate the ionic transport (either inward or outward) crossing the membrane to the intensity of the electric current applied, the time of current passage, and the charge per ion. It is important to realize that all the ions present at both sides of the skin compete to transport charge during iontophoresis.
Under the influence of a direct constant current, drug flux (Ji-mol/s) is given by
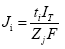
where ti is the transport number, IT isthe total current applied (Amperes), Zi the charge of the ion and F the Faraday’s constant (Coulombs/mol). Hence, an ion can function as a charge carrier provided it is small, fully charged, at high concentration, and is not protein bound.
Revere iontophoresis also extracts analytes by electroosmosis. Because skin carries a net negative charge at neutral pH, sodium ions in the interstitial fluid are major charge carrier through the skin. So the migration of these sodium ions makes a net convective solvent flow towards the cathode [33]. Th is solvent flow carries along with dissolved solutes allowing the transport of neutral, and especially, polar molecules that are extracted at the cathodal compartment [27]. However, the volume of solvent flow is directly proportional to the potential gradient between the electrodes [34, 35]. The electroosmosis transport is the basic mechanism underlying the reverse iontophoresis extraction of glucose, a neutral and polar substance. Furthermore, this convective flow reinforces the transport of cations while acting against that of anions. Th us, extraction of a cationic analyte will always be easier than that of an anion of similar physio-chemical and pharmacokinetics properties.
Studies on Therapeutic Drug Monitoring
The applications of reverse iontophoresis include general blood chemistry, glucose monitoring, the detection of diagnostic markers, and therapeutic drug monitoring. Studies on reverse iontophoresis to extract a variety of compounds are being investigated (Table 2). The use of reverse iontophoresis for noninvasive drug monitoring and pharmacokinetic profiling has been investigated for valproate, lithium, phenytoin, amikacin, caffeine, theophilline etc. The idea of applying reverse iontophoresis to extract molecules from the body was first reported in 1954 by Benjamin et al [36]. A low current density (0.5 mA/cm2) was applied using a metal on humans to extract sodium and potassium ions. In the latter part, Glikfeld et al have extracted three different molecules (clonidine, theophylline, and glucose) by the application of reverse iontophoresis [37]. Further, these molecules possess different properties such as one being a charged cation (clonidine), second one is partially charged anion (theophylline), and third one is a neutral polar molecule (glucose). They observed that the amount of molecules extracted across the skin was linearly correlated to the subdermal concentration, irrespective of the charge they possess.
| Drugs | Animal/membrane Model used | Inference | References |
|---|---|---|---|
| Amikacin | Full thickness rabbit ear skin | Cathodal extraction was much higher than anodal extraction | 23 |
| Amino acids | Full thickness porcine ear skin | All aminoacids were extracted iontophoretically to both electrodes | 53 |
| Amino acids | Six healthy subjects (20–51 Yrs) | Reverse iontophoresis could be utilized to extract 17 naturally occurring amino acids from the subdermal region | 55 |
| Lithium | Dermatomed pig ear skin | Good correlation between subdermal lithium and iontophoretic extraction fl ux was observed | 49 |
| Schizo-affective patients (23) | Lithium was proficiently extracted. Extraction fl ux of lithium was comparative to its serum concentrations | 25 | |
| Full thickness porcine ear skin | Lithium was efficiently extracted in solution and gel reservoir | 57 | |
| Phenylalanine | Dermatomed pig ear skin | Phenylalanine was efficiently extracted at the cathodal chamber and this approach can be applied for other amino acids | 17 |
| Phenytoin | Dermatomed pig ear skin | Ratio of extracted amount correlated well with subdermal concentration | 21 |
| Urea | Healthy subjects (24–47 Yrs) | Reverse iontophoresis can be employed to monitor urea concentrations in body and also in diagnosis of chronic kidney diseases | 51 |
The primary benefit of using reverse iontophoresis is that the pain and discomfort are reduced and the sampling can be drawn at any moment. The samples collected at regular interval could be sent and analyzed in the laboratory, thus provides incessantly monitoring of the sub cutaneous drug concentration. Further, the device used in reverse iontophoresis is miniaturized and can be easily transported, programmable and avoids hospitalization [38, 39].The technique is especially suitable in patients having difficulty of blood collection very frequently and those comes under specialized treatment like in case of cancer, AIDS, pregnantwomen, pediatric, geriatric and chronically ill individuals. Moreover, this technique could be useful for patients who are immovable and cannot impart problem with side effects of drug. In case of preterm infants the therapeutic drug monitoring is quite difficult and critical [40-42]. Blood sampling in this case preferably avoided as it increases risk of infection and finding a vein is very difficult due to prematurity [43,44]. In this case reverse iontophoresis could be the only possible option for the regular therapeutic drug monitoring. The application of reverse iontophoresis for noninvasive drug monitoring and pharmacokinetic profiling has been investigated for several molecules including valproate, lithium, phenytoin, amikacin, caffeine, and theophylline. The data observed form these studies suggests that iontophoresis can efficiently and noninvasively sample drugs of appropriate physicochemical and pharmacokinetic properties, perform drug monitoring and provide an estimation of the elimination (terminal) rate constant.
Th is technique could be utilized to assess the sub dermal concentration of charged and uncharged molecules by electromigration or electroosmosis [45, 46]. Moreover, the transport of these molecules is controlled by continuity and level of applied current. The evolution of sensitive and specific analytical tools which detects compound more precisely has extended the application of reverse iontophoresis. Although, the reverse iontophoresis is much efficient in extracting the solutes, the quantity of anlaytes could cross the skin layer is much low (10-1000 times lower than the subdermal concentration). However, the availability of sophisticated and sensitive analytical methods could overcome this issue.
Attention was focused on glucose for the application in glucose monitoring in diabetes. Th is approach carries an immense medical benefit for the noninvasive, continuous measurement of glucose, by increasing the frequency of measurements compared to conventional methods, and by avoiding invasive procedures to collect a blood sample or to place an implantable glucose sensor into the subcutaneous tissue. Several investigations have leads to the optimization of parameters for the reverse iontophoresis of glucose. in vitro and in vivo reverse iontophoresis experiments in non-diabetic subjects were promising and further research efforts led to the commercial development of the Glucowatch Biographer [47]. The principle involved in this device is the transport of glucose (neural molecule) by electroosmosis and is proportional to the applied current. The glucowatch biographer utilizes execution of biosensing the amount of glucose transported across the skin layer [47, 48]. The potential of glucowatch biosensor was evaluated in 92 human subjects. Clinical results with this device showed close tracking of blood glucose over a period of 12 h and a good correlation between glucowatch biographer reading and blood glucose was observed, although the biographer reading lag behind blood glucose level values by 18 min. The study concluded that the automatic, frequent, and non invasive measurement obtained with the glucowatch biographer provide substantially more information about glucose levels than the fingerstick method [3]. In another attempt, the precision and accuracy of the glucowatch (automated glucose biographer) was evaluated in 19 patients suffering from type 1 and type 2 diabetes mellitus. The glucose level was checked and compared with anticubital venous blood samples. The data observed indicate that the glucose concentration in both the methods were comparable [47]. Recently, attempts were made to develop a glucose biosensor based on carbon nanotubes material for reverse iontophoresis and was fabricated by immobilizing a mixture of glucose oxidase and multiwalled carbon nanotubes epoxy-composite, on a planar screen-printed carbon electrode [48].
The success of the reverse iontophoresis extraction technique in clinical studies has inspired various groups of research scientists and became the topic of current research.To extend the application of this technique, severalendogenous molecules such as lithium, urea, prostaglandin, lactate,phenylalanine, amino acids etc have been investigated.
Lithium, used to treat bipolar disorders, a low molecular weigh cationic molecule having a higher effective plasma concentration. in vitro studies were carried out to assess the feasibility of extracting this molecule. The results observed indicated excellent correlation and rapid extraction from a physiological buffer [49]. Studies were also carried out in bipolar patients showed the potential of reverse iontophoresis as a useful clinical tool for noninvasive monitoring of this drug. Monitoring of lithium was carried out in vivo by reverse iontophoresison 24 humans, using one arm as standard and other for test (area ~3.2 cm2). The results indicated efficient extraction of lithium from the sub dermal region. An excellent relationship between the extracted sample and serum concentration was reported [25].
Monitoring of urea in plasma could be used to investigate the kidney failure in patients with diabetes and hypertension [50]. in vivo studies were carried out in humans with impaired kidney function. The urea flux extracted by reverse iontophoresis was well correlated (r2=0.88) with analyte sub dermal levels and these are susceptible to time dependent changes in concentration.Wascotte et al optimized the procedure to monitor urea, in vitro and in vivo in healthy volunteers and patients with chronic kidney disease. Two cylindrical shaped glass cylinders was attached to each person’s forearm using silver-silver chloride electrode placed at 5 mm from surface of skin. A current intensity of 0.8 mA (for 2 h) was applied and samples were drawn and analyzed at every 30 min. The results were found to be promising as the extracted urea fluxes were diminuted with respect to time, both in healthy persons and patient of chronic kidney disease. The preliminary study demonstrated that monitoring of urea could be possible by the revere iontophoresis [50]. Studies were also carried out to monitor urea and potassium using porcine ear skin. Reverse iontophoresis was carried out for 6 h and the drug samples from the cathode chamber was analysed. The data observed indicated a good correlation between the sub dermal drug concentration and the amount of urea and potassium extracted [51]. The development of noninvasive techniques to monitor renal function would result in an improved therapy and a better quality of life for the patient [50].
Noninvasive lactatemia monitoring is of interest in the ease of critically ill patients as well as a marker of performance in sports training. Lactate, a relatively concentrated, small anion, constitutes a good candidate for reverse iontophoretic extraction. In fact, it is easily and rapidly extracted both in vitro and in vivo. The simultaneous in vivo extraction of chloride, and its possible role as an internal standard to calibrate lactate reverse iontophoretic fluxes, was also demonstrated [52]. In another attempt, extraction of lactate was attempted by reverse iontophoresis with the objective of determining the organ failure. Studies were carried out in 10 humans using screen printed electrodes coated with methyl cellulose gel. The gel was extracted and the lactate content was assessed and compared with the plasma lactate level. The results indicated a fair correlation [26].
Attempts were also made to extract different amino acids by reverse iontophoresis. Several in vitro and in vivo studies demonstrated that most amino acids could be extracted by reverse iontophoresis and could be analysed by the existing analytical techniques. The charged species were extracted in the opposite electrodes while the zwitter ionic species were extracted at both electrodes. The results indicated that the reverse iontophoresis have the potential of extracting different amino acids and can provide a reliable correlation [53]. Rapid extraction of amino acids by reverse iontophoresis after tape stripping was demonstrated [54]. Application of reverse iontophoresis was explored by assessing the feasibility of extracting phenylalanine an amino acid used to diagnose Phenylketonuria (metabolic disease in infants). The extraction was found to be rapid, easily detectable and linear (1-10 mM)correlation was observed between the amount of phenylalanine extracted and sub dermal concentration. In practice, this could provide much advantage in early detection of a metabolic disorder namely phenylketonuria [17].
In another study, the potential of reverse iontophoresis in extracting theophylline and caffeine was assessed using novel model which bear a resemblance to cutaneous barrier of neonate.Studies were carried out in full thickness and tape stripped porcine skin in vitro. The electric potential was applied for 5 h and the amount of drug extracted was assessed every hour using HPLC. The results indicated significant drug extraction following iontophoresis, suggesting the possibility of this technique to extract theophylline and caffeine [43].
Phenytoin, an antiepileptic drug possesses a very narrow therapeutic drug concentration. An investigation was carried out to extract phenytoin from the subdermal concentration, by reverse iontophoresis for monitoring of free drug in the body. Effect of drug concentration, protein concentration etc were investigated. Experiments were conducted in vitro using porcine ear skin using vertical diffusion cell. The results indicate that the extraction efficiency was influenced by change in protein concentration and drug concentration. However, the observation from this study points to the fact that the monitoring of phenytoin is very much possible using reverse iontophoresis [21]. Similarly, the technique of reverse iontophoresis for therapeutic drug monitoring was explored for valprioc acid as well. Studies were carried out to extract valproate, along with an internal standard (glutamate), in vitro. A constant current was applied for 5 h and the samples were withdrawn and analyzed. The results were found to be promising and a linear regression shows that correlation in-between fluxes and subdermal levels [20].
The application of reverse iontophoresis has been extended as a diagnostic tool. Mize et al have monitored the prostaglandin E2 in guinea pig by reverse iontophoresis, after inducing inflammation by regular transdermal iontophoresis of irritant drug compounds. The amount of PGE2 extracted on the anodal surface was monitored by radio immune assay. The study concludes that the prostaglandin E2 generated in response to transdermally applied drug irritants can be monitored by reverse iontophoresis [56].
Reverse iontophoresis of amikacin was carried out across rabbit skin in vitro by applying a constant current of 0.5 mA/cm2 for a period of 2 h. The amount of amikacin extracted across the skin at the cathode showed linear correlation with increase in time. Th is observation indicates that amikacin could be effectively extracted by reverse iontophoresis, and required further optimization [23].
Conclusion
Extensive study results suggest that reverse iontophoresis can efficiently and noninvasively sample analytes with the appropriate characteristics. Recent development in reverse iontophoresis substantiated its potential in the therapeutic drug monitoring. The development of glucowatch has established its great potential in the field of monitoring of glucose. Development of this method provides a great care for the diabetic patients. The importance of this device is greatly recognized not only for research purpose but also in other methods by which there will be an improvement in patient care and reduction in pain, discomfort. Few practical challenges remain to be resolved before this technology can be implemented for widespread use. Therapeutic drug monitoring can be accomplished by reverse iontophoresis and it is believed that in the future it will be proved as reliable in terms of miniaturization, sensitivity, specificity.In upcoming time, this method will be one of best noninvasive method that will be employed for monitoring of compounds in the body and likely to replace the process of blood sampling.
Conflicts of Interest
There are no conflicts of interest.
References
- Brown GR, Miyata M, McCormack JP. Drug concentration monitoring: an approach to rationale use. Clin Pharmacokinet. 1993; 24: 187–94.
- Leboulanger B, Guy RH, Delgado CMB. Reverse iontophoresis for noninvasive transdermal monitoring. Physiol Meas. 2004; 25: R35–R50.
- Kanikkannan N. Iontophoresis based transdermal delivery systems. BioDrugs. 2002; 16: 339-47.
- Rao G, Guy RH, Glikfeld P, et al. Reverse Iontophoresis: noninvasive glucose monitoring in vivo in humans. Pharm Res. 1995; 12: 1869-73.
- Pitzer KR, Desai S, Dunn T. Detection of hypoglycemia with the glucowatch biographer. Diabetes Care. 2001; 24: 881-5.
- Tierney MJ, Tamada JA, Potts RO. Clinical evaluation of the glucowatch biographer: a continual noninvasive glucose monitor for patients with diabetes. Biosens Bioelectron. 2001; 16: 621-29.
- Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002; 18: S49-S53.
- Murphy MG, Peck CC, Conner DP, et al. Transcutaneous theophylline collection in preterm infants. Clin Pharmacol Ther. 1990; 47: 427–34.
- Wang CY, Maibach HI. Why minimally invasive skin sampling techniques? A bright scientific future. Cutan Ocul Toxicol. 2011; 30: 1-6.
- Paulley Y, Delgado-Charro MB, White KAJ. Modelling formation of a drug reservoir in the stratum corneum and its impact on drug monitoring using reverse iontophoresis. Comput Math Method M. 2010; 11: 353-68.
- Suthakaran C, Adithan C. Therapeutic drug monitoring – concepts, methodology, clinical application and limitations. Health Adm. 19: 22-6.
- Gogtay NJ, Kshirsagar NA, Dalvi SS. Therapeutic drug monitoring in a developing country: an overview. Br J Clin Pharmacol. 1999; 48: 649-54.
- Anroop B, Ghosh B, Parcha V, et al. Transdermal delivery of atenolol: effect of prodrugs and iontophoresis. Curr Drug Deliv. 2009; 6: 280-90.
- Nair A, Reddy C, Jacob S. Delivery of a classical antihypertensive agent through the skin by chemical enhancers and iontophoresis. Skin Res Technol. 2009; 15: 187-94.
- Batheja P, Thakur R, Michniak B. Transdermal iontophoresis. Expert Opinion Drug Deliv. 2006; 3: 127-38.
- Sieg A, Wascotte V. Diagnostic and therapeutic applications of iontophoresis. J Drug Target. 2009;17: 690-700.
- Merino V, Lopez A, Hochstrasser D, et al. Noninvasive sampling of phenylalanine by reverse iontophoresis. J Control Release. 1999; 61: 65–9.
- Camille CB, Jean PS, Guy RH, et al. Reverse iontophoresis of amino acids identification and separation of stratum corneum and subdermal sources in vitro. 2009; 26: 2630-38.
- Peck CC, Lee K, Becker CE. Continuous transepidermal drug collection: basis for use in assessing drug intake and pharmacokinetics. J Pharmacokinet Biopharm. 1981; 9: 41–58.
- Delgado-Charro MB, Guy RH. Transdermal reverse iontophoresis of valproate: a noninvasive method for therapeutic drug monitoring. Pharm Res. 2003; 1508–13.
- Leboulanger B, Guy RH, Delgado-Charro MB. Noninvasive monitoring of phenytoin by reverse iontophoresis. Eur J Pharm Sci. 2004; 22: 427-33.
- Sieg A, Guy RH, Delgado-Charro MB. Reverse iontophoresis for noninvasive glucose monitoring: the internal standard concept. J Pharm Sci. 2003; 92: 2295-302.
- Nicoli S, Santi P. Transdermal delivery of amino glycosides: amikacin transport and iontophoretic noninvasive monitoring. J Control Release. 2006; 111: 89-94.
- Glikfeld P, Cullander C, Hinz RS, et al. A new system for in vitro studies of iontophoresis. Pharm Res 1988; 5: 443–46.
- Leboulanger B, Aubry JM, Bondolfi G, et al. Lithium monitoring by reverse iontophoresis in vivo. Clin Chem. 2004; 50: Y2091-Y100.
- Ching TS. Simultaneous transdermal extraction of glucose and lactate from human subjects by reverse iontophoresis. Int J Nanomedicine. 2008; 3: 211- 33.
- Phipps JB, Gyory JR. Transdermal ion migration. Adv Drug Deliv Rev. 1992; 9: 137–76.
- Kalia YN, Naik A, Garrison J, et al. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004; 56: 619-58.
- Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev.1992; 9: 201-37.
- Pikal MJ .Transport mechanisms in iontophoresis. I. A theoretical model for the effect of electroosmotic flow on flux enhancement in transdermal iontophoresis. Pharm Res. 1990; 9: 118-26.
- Pikal MJ, Shah S. Transport mechanisms in iontophoresis. III. An experimental study of the contributions of electroosmotic flow and permeability change in transport of low and high molecular weight solutes. Pharm Res. 1990; 7: 222-31.
- Santi P and Guy RH. Reverse iontophoresis - parameters determining electroosmotic flow: I. pH and ionic strength. J Control Release. 1996; 38: 159-65.
- Santi P, Guy RH. Reverse iontophoresis - parameters determining electroosmotic flow: II. Electrode chamber formulation. J Control Release. 1996; 42: 29-36.
- Marro D, Kalia YN, Delgado-Charro MB, et al. Contributions of electromigration and electroosmosis to iontophoretic drug delivery. Pharm Res. 2001; 18: Y1701-Y08.
- Sage BH, Riviere JE. Model systems in iontophoresis - transport efficacy. Adv Drug Deliv Rev. 1992; 9: 265-87.
- Benjamin FB, Kempen R, Mulder AG, et al. Sodium-potassium ratio of human skin as obtained by reverse iontophoresis. J Appl Physiol. 1954; 6: 401-07.
- Glikfield P, Hinz RS, Guy RF. Noninvasive sampling of biological fluids by iontophoresis. Pharm Res. 1989; 11: 988–90.
- Merino V, Kalia YN, Guy RH. Transdermal therapy and diagnosis by iontophoresis. Trends Biotechnol. 1997; 15: 288–90.
- Gross AS. Best practice in therapeutic drug monitoring. Br J Clin Pharmacol. 1998; 46: 95–9.
- Langer VS. Minimal handling protocol for the intensive care nursery. Neonatal Netw. 1990; 9: 23–7.
- Barrett DA, Rutter N. Transdermal delivery and the premature neonate. Crit Rev Ther Drug Carrier Syst. 1994; 11: 1–30.
- Glikfeld P, Hinz RS, Guy RH. Noninvasive sampling of biological fluids by iontophoresis. Pharm Res. 1989; 6: 988-90.
- Sekkat N, Naik A, Kalia YN, et al. Reverse iontophoretic monitoring in premature neonates: feasibility and potential. J Control Release. 2002; 81: 83–9.
- Sekkat N, Kalia YN, Guy RH. Transdermal drug delivery to premature neonates II: passive diffusion versus iontophoresis. Pharm Res. 2001; 18: 1006-11.
- Marro D, Kalia YN, Delgado-Charro MB, et al. Contributions of electromigration and electroosmosis to iontophoretic drug delivery. Pharm Res. 2001; 18: 1701-8.
- Sieg A, Guy RH, Delgado-Charro MB. Electroosmosis in transdermal iontophoresis: implications for noninvasive and calibration-free glucose monitoring. Biophys J. 2004; 87: Y3344-Y50.
- Sang YR, Suk C, Gwanpyo K, et al. Clinical experience of an iontophoresis based glucose measuring system. J Korean Med Sci. 2007; 22: 70-3.
- Tai-Ping S, Hsiu-Li S, Congo TSC, et al. Carbon nanotube composites for glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. Int J Nanomedicine. 2010; 5: 343–49.
- Leboulanger B, Fathi M, Guy RH, et al. Reverse iontophoresis as a noninvasive tool for lithium monitoring and pharmacokinetic profiling. Pharm Res. 2004; 21: 1214-22.
- Valentine W, Eric R, Ana S, et al. Noninvasive diagnosis and monitoring of chronic kidney disease by reverse iontophoresis of urea in vivo. Eur J Pharm Biopharm. 2008; 69: 1077–82.
- Valentine W, Delgado-Charro MB, Eric R, et al. Monitoring of urea and potassium by reverse iontophoresis in vitro. Pharm Res. 2007; 24: 1-7.
- Nixon S, Sieg A, Delgado-Charro MB, et al. Reverse iontophoresis of L-lactate: in vitro and in vivo studies. J Pharm Sci. 2007; 96: 3457-65.
- Anke S, Fabienne J, Marc F, et al. Extraction of amino acids by reverse iontophoresis invitro. Eur J Pharm Biopharm. 2008; 70: 908-13.
- Bouissou CC, Sylvestre JP, Guy RH, et al. Reverse iontophoresis of amino acids: identification and separation of stratum corneum and subdermal sources in vitro. Pharm Res. 2009; 26: 2630-8.
- Anke S, Fabienne J, Marc F, et al. Extraction of amino acids by reverse iontophoresis invivo. Eur J Pharm Biopharm. 2009; 72: 226-31.
- Mize NK, Butterly M, Daddona P, et al. Reverse iontophoresis: monitoring prostaglandin E2 associated with cutaneous inflammation in vivo. Exp Dermatol. 1997; 6: 298-302.
- Valentine W, Laboulanger B, Guy RH, et al. Reverse iontophoresis of lithium: electrode formulation using a thermoreversible polymer. Eur J Pharm Biopharm. 2005; 59: 237-40.