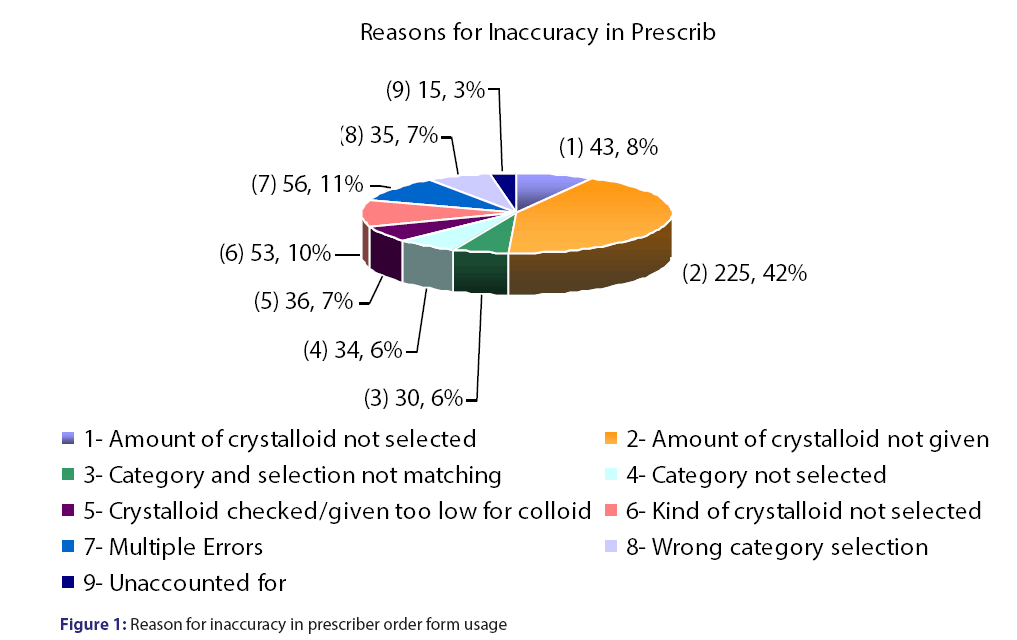The Impact of Criteria for Use and a Prescriber Order From on Albumin Utilization
Published: 20-Aug-2017
Citation: Tucker C. The Impact of Criteria for Use and a Prescriber Order Form on Albumin Utilization. J Basic Clin Pharma 2017;8:255-258.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Purpose: Fluid management is fundamental to the care of critically ill patients. Albumin solutions are protein colloids that are oncotically equivalent to human plasma. These solutions are commonly administered to increase or maintain intravascular volume. Within the critical care setting, albumin is often used for several reasons including distributive and hemorrhagic shock, cerebral ischemia and maintenance of cerebral perfusion pressure, and management of postoperative hypotension. The choice of whether to use crystalloids or colloids for these indications continues to be debated despite the lack of adequate evidence or guidelines to support its use. Jackson Memorial Hospital (JMH) implemented albumin usage criteria in concordance with the University Health Consortium (UHC) guidelines in 2007 in order to establish evidence based criteria for use and promote a cost-effective approach without compromising patient care. The study was conducted in two phases, a pre-implementation phase and a post-implementation phase. Methods: This retrospective cohort study was conducted from November 2011 to November 2012 to assess adherence with the revised usage guidelines and prescriber order form for albumin. A data collection tool was developed to collect the following: age, sex, physician and specialty/service, length of stay, indication(s), contraindication(s) to non-colloid use (if any), albumin concentration, dose, frequency, dose volume, number of doses, adverse event associated with albumin, and use of other concurrent therapy. Each instance of albumin use will be assessed for its indication then correlated with the current guidelines and literature. Results: The primary indications for albumin use were hypotension/hypovolemia, cerebral ischemia/perfusion, low serum albumin and cirrhosis/paracentesis. There were 953 doses evaluated. Appropriateness of colloid utilization post implementation was 54.4%. When used for hypotension/hypovolemia, the appropriateness was 49% and 67% received crystalloid prior to colloid use. With implementation of the protocol for colloid use, the cost savings for albumin expenditure was approximately $400,000. Conclusion: Implementation of revised guidelines and dedicated order form was deemed necessary to promote more appropriate albumin utilization. Despite complete use of order form only being approximately 50%, its implementation was able to effectively decrease albumin expenditure.
Keywords
Hypotension, hypovolemia, cerebral ischemia
Background
The administration of intravenous fluids is a universally accepted intervention for the resuscitation of acutely ill patients. Hypovolemia is associated with significant pathophysiological changes that can lead to organ dysfunction and profound organ failure. [1,2] Ensuring that patients are adequately volume resuscitated is fundamental to providing appropriate cardiac stability and hemodynamic support. Crystalloids contain water soluble molecules that are isotonic to human plasma in an effort to maintain intravascular volume. However, in many acutely ill patients, increased capillary permeability and depletion of intravascular proteins can cause fluid to leave the intravascular space and accumulate in the interstitial space. [3] Albumin solutions are protein colloids that are oncotically equivalent to human plasma. It is theorized that the use of colloids such as albumin can provide superior volume resuscitation by increasing the oncotic pressure to retain fluid in the intravascular space. Oncotically active solutions are large insoluble molecules that remain in the intravascular space and are not affected by the changes in capillary permeability. [4] However, in critical illness, due to the rate of capillary leak and other physiological changes, the benefit of retaining colloid solutions in the intravascular space is diminished. Several trials have examined the utility of colloid in the resuscitation and hemodynamic support of volume depleted patients without demonstrating any significant benefit for patient outcomes. [1,5-7]
Standardized order sets are taking center stage as an invaluable clinical decision support tool with wide-ranging benefits for both patients and healthcare organizations. Implementation of standardized order sets, templates, or protocols are commonly used to improve compliance with recommended processes of care. Order sets provide straightforward clinical decision support within computerized provider order entry systems. They are designed to reflect current guidelines and best practice standards to help guide appropriate utilization of medications and other resources. The impact of such tools on resource use appears more variable, depending in part on the clinical area or type of care targeted. Given the massive inappropriate use of albumin, order sets can provide guidance to promote appropriate utilization.
In 2000, the UHC revised their 1993 Albumin, non-protein Colloid, and Crystalloid solutions guidelines to aid in the development of policies and procedures to ensure the appropriate use of albumin and other plasma expanders. Albumin has FDA approved indications for a variety of disease states. In many clinical settings when compared to crystalloids, albumin offers no additional advantages and may increase the risk of adverse events. Jackson Memorial Hospital (JMH) implemented albumin usage criteria in concordance with the UHC guidelines in 2007 to increase education and promote evidence-based use of albumin. Albumin utilization at JMH remained high, accounting for over one million dollars annually. According to a medication use evaluation conducted prior to the implementation of the revised guidelines and order form demonstrated that 60-70% of albumin utilization was inappropriate. The purpose of this study was to assess the ability of a dedicated prescriber order form to promote appropriate utilization of albumin.
Methodology
This retrospective cohort was conducted from November 2011 to November 2012. For each instance of albumin use specific data was collected depending on the indication observed. Albumin utilization was evaluated by each instance entered into the pharmacy database. A data collection tool was developed to collect the following: Age, sex, physician and specialty/service, length of stay, indication(s), contraindication(s) to non-colloid use (if any), albumin concentration, dose (g), volume (mL) frequency, number of doses, adverse event(s) associated with albumin, and use of other concurrent therapy. Each instance of albumin use was assessed for its indication then correlated with the current guidelines and literature.
Descriptive statistics were used to analyze the results from the DUE, such as patient demographics, albumin use per indication, attending on key plate, physician specialty, location and if crystalloids were administered prior to albumin. A cost analysis was performed to determine the financial impact of appropriate utilization of albumin.
Results
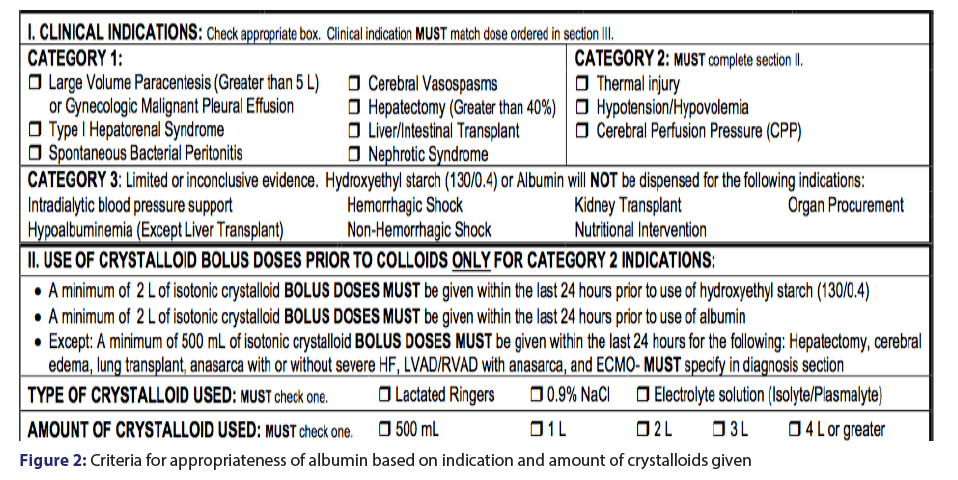
Prescriber order form utilization was 96%, however, the form was only completed at a rate of 57.6%. Prescriber accuracy with the order from was 45%, with the most common reason for inaccurate and incomplete use of the order form were crystalloids not given in Table 1 and Figure 1. A total of 398 patients were evaluated accounting for 953 doses [Table 1]. The majority (62%) of the doses were used for hypotension/hypovolemia and liver/intestinal transplant (17.4%) [Table 2]. Appropriateness was determined if the indication for albumin use was for an unapproved indication per our revised guidelines or if the appropriate amount of fluid was administered prior to albumin use [Figure 2]. The inappropriate use of albumin post-implementation was 45% with the majority due to the indication of cerebral perfusion and hypotension/hypovolemia [Table 2].
| Patient Characteristics | Compliance with Albumin Form | |||
|---|---|---|---|---|
| Gender | Form Usage | |||
| Female | 150 | Yes | 919 | 96% |
| Male | 248 | No | 34 | 4% |
| Total # of Patients | 398 | Form Filled Out Completely by Prescriber | ||
| # of Doses | Yes | 549 | 57.60% | |
| ICU | 709 | No | 404 | 42.40% |
| Diagnosis | 202 | 50% | ||
| Non-ICU | 244 | Category | 80 | 20% |
| Kind of Crystalloid | 167 | 41.30% | ||
| Total # of Doses | 953 | Amount of Crystalloid | 80 | 20% |
Table 1: Patient demographics and prescriber order form compliance
| Reason for Use | Appropriate n (%) | Inappropriate n (%) | Total |
|---|---|---|---|
| Large Volume Paracentesis | 37 (80%) | 9 (20%) | 46 |
| Type I Hepatorenal Syndrome | 33 (66%) | 17 (34%) | 50 |
| Spontaneous Bacterial Peritonitis | 10 (38.5%) | 16 (61.5%) | 26 |
| Cerebral Perfusion Pressure | 10 (15%) | 58 (85%) | 68 |
| Hepatic Resection | 15 (88.2%) | 2 (11.8%) | 17 |
| Liver/Intestinal Transplant | 93 (90.3%) | 10 (9.7%) | 103 |
| Nephrotic Syndrome | 4 (25%) | 12 (75%) | 16 |
| Thermal Injury | 27 (96%) | 1 (4%) | 28 |
| Hypotension/Hypovolemia | 289 (48.9%) | 302 (51%) | 591 |
| Intradialytic BP Support | 0 | 5 (100%) | 5 |
| Organ Procurement | 0 | 3 (100%) | 3 |
| Total | 518 (54.4%) | 435 (45.6%) | 953 |
Table 2: Appropriateness of colloid utilization based on established protocol
Implementation of updated albumin usage guidelines and creation of a prescriber order form allowed for a reduction in albumin utilization and significant cost savings. Prior to the protocol annual albumin expenditure averaged $1.27 million each year over the last three years. After implementation of the guidelines and order form albumin expenditure decreased to approximately $866,000. Without the albumin protocol and, albumin expenditure would have been approximately $1.4 million, allowing for a cost savings of approximately $400,000.
Discussion
Albumin utilization at JMH was steadily increasing over several years. The theory of colloids being superior to crystalloids due to the increased oncotic pressure is not substantially supported, yet clinicians that use this theory as their primary justification for albumin utilization can incur significant cost to healthcare systems. A meta-analysis evaluated albumin utilization demonstrated a six percent increase in absolute risk of death in patients they received albumin compared to crystalloids. [6] However, a 2006 meta-analysis demonstrated no difference in the risk of death between albumin and crystalloids. [1] The SAFE trial also determined that albumin has no significant benefit on mortality, length of stay, pulmonary or renal function when compared to normal saline. [5] In 2013, the CRISTAL trial evaluated the use of colloids versus crystalloids in hypovolemic shock. Patients received either a colloid or a crystalloid for all their fluid interventions. There was no significant difference observed between the patients that received crystalloids or colloids. [7] These conflicting results within the literature have caused uncertainty pertaining to the proposed benefit of albumin containing solutions. [7] Within the critical care setting, albumin is used for several indications despite the lack of definitive evidence. [8-11] Crystalloids can sufficiently replenish the intravascular volume without the adverse effects observed with albumin, such as anaphylaxis and infection. [12] For certain patient populations, such as traumatic brain injury, thermal injury and distributive shock, the administration of albumin have been associated with increased length of stay and mortality. [5] In addition, albumin administration to cardiac surgery patients provided no additional benefit when compared to crystalloids. [9]
The use of a colloidal alternative to albumin has been previously explored with a variety of products such as dextrans, gelatins and hydroxyl-ethyl starches. Synthetic colloids are more cost effective than albumin but are associated with alterations in coagulation, inflammatory markers and organ function. HES are synthetic colloids that are used for plasma volume expansion. Coagulation abnormalities and nephrotoxicity are adverse events typically observed with older generation HES products. The older generations of HES solutions have higher molecular weight, which has been shown to have a more pronounced effect on clotting factors and platelet activity. HES 130/0.4, the newest HES approved by the FDA, is a low molecular weight starch theorized to have significantly less effect on clotting factors and platelet function. There is conflicting literature in regards to the safety and efficacy of HES 130/0.4, questioning its ability to be a suitable alternative to albumin. [10] HES 130/0.4 now contains warnings stating it should not be used in critically ill patients due to increased risk of death and renal replacement therapy, When HES 130/0.4 is compared to crystalloids for fluid resuscitation, HES 130/0.4 provided no significant difference in mortality, but was associated with higher incidence of renal replacement therapy and more adverse events. [13-19] In severe sepsis, HES 130/0.4 was related to an increased risk of death at 90 days and more severe bleeding events than lactated ringers. [17] Fluid balances and volumes did not differ between the groups which questions the utility of HES 130/0.4 in severe sepsis. [17-19] The CRYSTMAS study, evaluated HES 130/0.4 versus normal saline in patients with severe sepsis as the primary resuscitation fluid. Significantly less volume was needed to achieve hemodynamic stability with HES 130/0.4 when compared to normal saline without any difference noticed in adverse events. [19] Bayer et al. evaluated colloid therapy (HES 130/0.4 and 4% gelatin) versus crystalloids in patients with severe sepsis. Given as the primary resuscitation fluid, the patients in the colloid group trended to more renal impairment and higher ICU mortality when compared to the crystalloid group. [20,21]
When utilized as the sole resuscitation fluid, HES 130/0.4 offers no benefit over crystalloids and may increase adverse events. However, HES 130/0.4 and other colloids are typically not used as a solitary resuscitation fluid but in conjunction with crystalloids. Crystalloids remain the resuscitation fluid of choice reserving colloids to augment resuscitation efforts. In many of the studies that evaluate HES 130/0.4 for fluid resuscitation utilized several liters per patient. None of the current literature evaluates the use of the combination of colloids with crystalloids for fluid resuscitation. Colloid administration in many of the studies is not consistent with current recommendations that advocate supplementation with colloids after sufficient amount of crystalloids have been administered. The promotion of crystalloids as the primary resuscitation fluid for these indications is critical, but the use of HES 130/0.4 offered a more cost effective alternative to the use of albumin. During our study period, data on adverse events were not routinely collected, however none were observed during this study, consistent with the CRYSTMAS study.
As part of the prescriber order form, the patient was required to receive at least 2 liters of crystalloids within a 24 hour prior to a dose of a colloid. For the indications of hypotension/hypovolemia and nonhemorrhagic shock, crystalloids are considered first line. The prescriber order form required selection of the indication that were divided into three categories 1 to 3, with category 1 being indications with most evidence and category 3 being the indications with the least evidence. Doses of colloids were also specified based on the indication. Using the order form to direct therapy to be more appropriate contributed to the reduction of albumin expenditure. The use of HES 130/0.4 as a part of the order provided a more cost effective option for prescribers. Given to overwhelming safety concerns, HES 130/0.4 was eventually removed from the order set. In the studies that evaluated the utility HES 130/0.4 used it as the sole resuscitation fluid resulting in patients receiving several liters of HES 130/0.4. During the course of our study it was recommended crystalloids were used in conjunction with any colloid therapy, limiting the cumulative dose of colloids received. Current recommendations do not advocate colloids as the sole resuscitation fluid and the amount of HES/130.04 patients received could possibly be link to adverse events. Without HES 130/0.4 as an alternative, the order form advocated for more appropriate use of colloids, still resulting in a overall decrease in expenditure at JMH.
Conclusion
Implementation of revised guidelines and dedicated order form was deemed necessary to promote more appropriate albumin utilization. Its implementation was able to effectively increase albumin appropriateness and decrease albumin expenditure. For the indications such as hypotension/hypovolemia and non-hemorrhagic shock, the order form was able to help advocate crystalloid administration prior to colloid use resulting in a decrease in total albumin costs. Criteria of use and indications found in guidelines and order sets can easily be incorporated into computerized physician order entry and clinical support tools in the electronic medical record.
REFERENCES
- Finfer S, Bellomo R, Boyce N.Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from saline versus albumin fluid evaluation (SAFE) study. BMJ 2006;333:1044.
- Boldt J, Scholhorn T, Mayer J. The Value of an Albumin-Based Intravascular Volume Replacement Strategy in Elderly Patients Undergoing Major Abdominal Surgery. Anesth Analg 2006;103:191-923.
- Meyer P, Pernet P, Hejblum G. Haemodilution Induced by Hydroxyethyl Starches 130/0.4 is Similar in Septic and Non-Septic Patients. Acta Anaesthesiol Scand 2008;52:229-35.
- Haynes GR, Navickis RJ, Wilkes MM. Albumin administration-what is the Evidence of Clinical Benefit? A Systematic Review of Randomized Controlled Trials. Eur J Anaesthesiol 2003;20:771-93.
- Finfer S, Bellomo R, Boyce N.Saline or Albumin for Fluid Resuscitation in Patients with Traumatic Brain Injury. N Engl J Med 2007;357:874-84.
- Choi PT, Yip G, Quinonez LG, Cook DJ. Crystalloids vs. Colloids in Fluid Resuscitation: A Systematic Review. Crit Care Med 1999;27:200-10.
- Annane D, Siami S, Jaber S. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 2013;310:1809-17.
- Wilkes MM, Navickis RJ. Patient Survival after Human Albumin Administration. Ann Intern Med 2001;135:149-64.
- Sade RM, Stroud MR, Crawford FA, Kratz JM, Dearing JP. A Prospective Randomized Study of Hydroxyethyl Starch, Albumin, and Lactated Ringer’s Solution as Priming Fluid for Cardiopulmonary Bypass. J Thorac Cardiovasc Surg 1985;89:713-22.
- Franz A, Braunlich P, Gamsjager T. The Effects of Hydroxyethyl Starches of Varying Molecular Weights on Platelet Function. Anesth Analg 2001;92:1402-7.
- Fox DL, Vermeulen LC. Albumin, Nonprotein, Colloid and Crystalloid Solutions. University HealthSystem Consortium. Oak Brook, Illinois, USA. 2000.
- American Thoracic Society. Evidence-based Colloid Use in the Critically Ill: American Thoracic Society Consensus Statement. Am J Respir Crit Care Med2004;170:1247-59.
- Wong F. Drug Insight: The Role of Albumin in the Management of Chronic Liver Disease. Gastroenterol and Hepatol 2007;4:43-51.
- Sort P, Navasa M, Arroyo V.Effect of Intravenous Albumin on Renal Impairment and Mortality in Patients with Cirrhosis and Spontaneous Bacterial Peritonitis.N Engl J Med 1999;341:403-9.
- Salerno F, Badalameenti S.Repeated Paracentesis and IV Albumin Infusion to treat ‘Tense’ Ascites in Cirrhotic Patients. J of Hepatol 1987;5:102-8.
- Chalasani N. Effects of Albumin/Furosemide Mixtures on Responses to Furosemide in Hypoalbuminemic Patients. J Am Soc Nephrol2001;12:1010-6.
- Myburgh JA, Finfer S, Bellomo R. Hydroxyethylstarch or saline for fluidresuscitation in intensive care. N Engl J Med 2012;367:1901-11.
- Perner A, Haase N, Guttormsen AB. Hydroxyethyl starch 130/0.42 versus Ringer'sacetate in severesepsis. N Engl J Med 2012;367:124-34.
- Gattas DJ, Dan A, Myburgh J. Fluidresuscitation with 6% hydroxyethylstarch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med 2013;39:558-68.
- Guidet B, Martinet O, Boulain T. Assessment of hemodynamic efficacy and safety of 6% hydroxyl-ethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit Care 2012;16:R94.
- Bayer O, Reinhart K, Kohl M. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med2012;40:2543-51.