Synthesis of Some New 3-Coumarinoyl Pyridinium and Quinolinium Bromides for their Antimicrobial Activity
- *Corresponding Author:
- Mahesh Attimarad
College of Clinical Pharmacy, King Faisal University, Al-Hasa, KSA
E-mail: mattimarad@gmail.com
Date of Received: 20-01-2010
Date of Modified: 29-01-2010
Date of Accepted: 02-02-2010
Available Online: 15-02-2010
Abstract
3-Bromo acetyl coumarins (4a-c) on treatment with methyl and ethyl es- ters of nicotinic acid, isonicotinic acid (5a-d) gave 3-coumrinoyl pyridinium bromides (7a-l) and with quinoline (6) gave 3-coumarinoyl quinolinium bromides (8a-c). the structures of the final compounds were established by their spectral data. the antimi- crobial activitiy of all the newly synthesized test compounds were evaluated and some of them, showed good antibacterial activity.
Keywords
Coumarins, Antimicrobial Activity
Introduction
Coumarins are known to possess an array of biological activities such as photodynamic [1], analgesic [2], antimicrobial [3], antifungal [4] and antiviral [5] and are used in treatment of vitiligo, psoriasis and other dermal diseases [6-8]. Incorporation of small heterocyclic system at third position on coumarins are known to exhibit variation in their biological activity [9]. Series of heteroaryl coumarins have already been synthesized in our laboratory with five membered pyrazoline and thiazole substitution at third position on coumarins and were evaluated for their anti-inflammatory, analgesic and antimicrobial activity [10,11]. This prompted for further synthesis of useful six membered heterocyclic substitutions on coumarins.
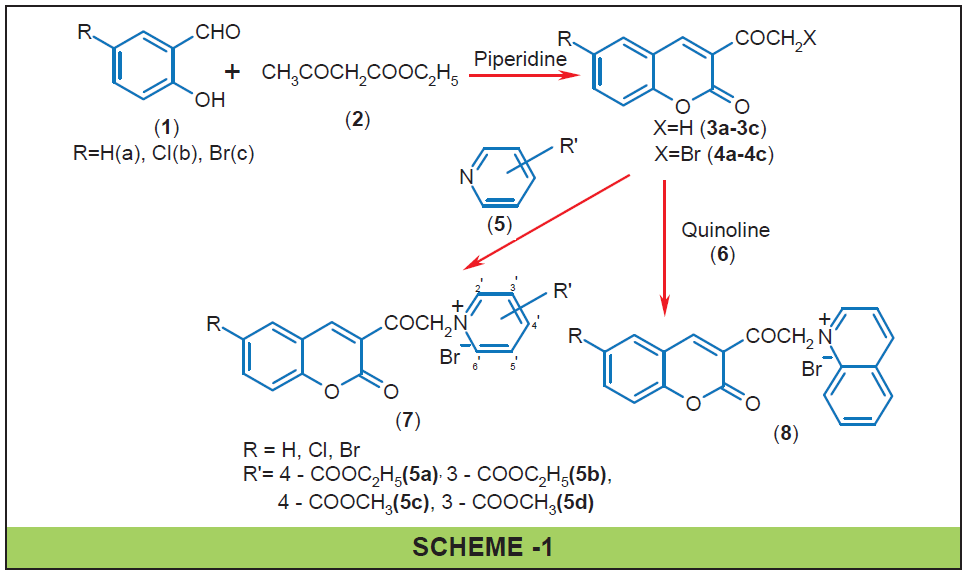
3-Acetyl coumarins (3a-c) were synthesized by condensation of salicyladehyde [1] and ethyl acetoacetate [2], which was brominated to yield 3-bromoacetyl coumarins (4a-c). 3-Bromo acetyl coumarins (4a-c) on treatment with methyl and ethyl esters of nicotinic acid, isonicotinic acid (5a-d) yielded 3-coumarinoyl pyridinium bromides (7a-l) and with quinoline (6) yielded 3-coumarinoyl quinolinium bromides (8a-c). IR, NMR and Mass spectral data supported the structural assignments of 7a-l and 8a-c.
Melting points of the test compounds were determined by using Thiele’s melting point apparatus and were found uncorrected. The IR spectra were run on Shimadzu FT-IR spectrophotometer in KBr pellets. 1H NMR was obtained using JEOL AMX-400, FT-NMR 400MHz in CDCl3/DMSO-d6 as solvent and Tetra Methyl Silane (TMS) as an internal standard. Mass spectra of few of the test compounds were recorded by JEOL JMS-300 spectrometer at 70eV by electron impact ionization.
3-Bromoacetyl coumarin (4a)
3-Acetylcoumarin 3a (18.8 g, 0.1 mol) in glacial acetic acid (150 ml) was taken in a conical flask and to it a mixture of bromine (5.12 ml, 0.1 mol) in glacial acetic acid (20 ml) was added with stirring for 30 min. at room temperature. The mixture was stirred at room temperature for another 3 hr. It was then poured into ice-cold water and the ester 4a separated out as solid was filtered, washed with water and dried. The product was further recrystallized from chloroform (yield, 20.2 g 76%; m. p. 165°). Other 3-bromoacetyl coumarins (4b-c) were prepared similarly (Scheme-1).
Ethyl isonicotinate (5a)
Isonicotinic acid (12.3 g, 0.1 mol), ethanol (30 ml, 1 mol) and concentrated sulphuric acid (2.2 ml, 0.04 mol) were taken in 250 ml round bottom flask and refluxed for 6 hr. The reaction mixture was then poured into ice-cold water and the oil separated on basification with sodium carbonate was extracted with 3 × 50 ml portions of ether. Combined extracts were washed twice with saturated solution of sodium bicarbonate and then with water. Pure ester (5a) was obtained by distilling off the ether (yield, 76%; m. p. 223-224°). Other methyl and ethyl esters of isonicotinic acid and nicotinic acid (5b-d) were similarly prepared.
3-Coumarinoyl (4’-ethoxy carbonyl) pyridinium bromide (7a)
To a solution of 3-bromoacetylcoumarin 4a (0.03 mol) in dry toluene (100 ml) was added ethyl isonicotinate 5a (0.0315 mol) and the mixture was heated at reflux temperature for 2 hr. The solution was cooled to room temperature and was maintained at this temperature for 4 to 5 hours. The resultant pyridinium bromide (7a) was filtered out and washed with hot toluene. It was then dried and crystallized from ethanol: n-hexane (yield, 85%; m. p. 210°). IR (KBr) : 3402 cm-1 (N-CH2), 3022 cm-1 (ArC-H), 2958 cm-1 (C-H), 1730 cm-1 (C=O of ester), 1687 cm-1 (C=O of lactone), 1610cm-1 (C=N), 1552, 1487, 1448 cm-1 (ArC=C), 1247cm-1 (C-N). 1H NMR (CDCl3): δ 1.47 (3H, t, CH2CH3), 4.53 (2H,q, CH2CH3), 7.13 (1H, d, ArH), 7.28-7.33 (3H, m, CH2 and ArH), 7.62 (1H, t, ArH), 7.71 (1H, d, ArH), 8.50 (2H, d, J=8.1Hz ArH3’5’), 8.77 (1H, s, ArH), 9.91 (2H, d, J=8.1Hz ArH2’6’).
Other coumarinoyl pyridinium bromides (7b- 7l) and coumarinoyl quinolinium bromides (8a-8c) were similarly synthesized (Scheme-1).
7c: IR ( KBr) : 3407 cm-1 (N-CH2), 3006 cm-1 (ArC-H), 2906 cm-1 (C-H), 1735 cm-1 (C=O of ester), 1703 cm-1 (C=O of lactone), 1612 cm-1 (C=N), 1560, 1463, 1417 cm-1 (ArC=C), 1292 cm-1 (C-N), 752 cm-1 (C-Cl). 1H NMR (CDCl3) : δ 1.47 (3H,t, CH2CH3), 4.55 (2H, q, CH2CH3), 6.79 (2H,s, ArH), 7.42 (1H, d, ArH), 7.70 (1H, m, ArH), 7.89 (1H, s, ArH), 8.55 (2H, d, ArH3’5’), 8.62 (1H,s, ArH), 9.59 (2H, d, ArH2’6’). Mass: m/z - 452 (M+), 403 (Base peak), 406, 372, 220, 206, 193, 165, 150, 137, 79. 7k: IR ( KBr) : 3435 cm-1 (N-CH2), 3003 cm-1 (ArC-H), 2922 cm-1 (C-H), 1732 cm-1 (C=O of ester), 1681 cm-1 (C=O of lactone), 1600 cm-1 (C=N), 1548, 1471 cm-1 (ArC=C), 1300 cm-1 (C-N), 661 cm-1 (C-Br). 1H NMR (DMSO-d6): δ 4.02 (3H, s, CH3), 6.40 (2H, s, CH2), 7.65 (1H, d, ArH), 7.90 (1H, d, ArH), 8.25 (1H, s, ArH), 8.65 (2H, d, ArH3’5’), 8.95 (1H, s, ArH), 9.14 (2H, d, ArH2’6’).
8a: IR ( KBr) : 3405 cm-1 (N-CH2), 3000 cm-1 (ArC-H), 2825 cm-1 (C-H), 1695 cm-1 (C=O of lactone), 1604 cm-1 (C=N), 1554, 1446 cm-1 (ArC=C), 1247 cm-1 (C-N). 1H NMR (DMSOd6) : δ 6.73 (2H, s, CH2), 7.51 (1H, t, ArH), 7.63 (1H, d, ArH), 7.89 (1H, t, ArH), 8.05-8.12 (2H, m, ArH), 8.15–8.24 (1H, m, ArH), 8.30-8.34 (1H, m, ArH), 8.54 (2H, t, ArH), 8.91 (1H, s, ArH), 9.44 (1H, d, ArH), 9.51 (1H, d, ArH).
Antimicrobial Activity
All the synthesized test compounds were qualitatively screened for their antimicrobial activity against Bacillus subtilis (B. s.), Escherichia coli (E. c.), Pseudomonas aeruginosa (P. a.), Staphalococcus aureus (S. a.) using agar diffusion method [12]. All the test compounds were dissolved in dimethylsulphoxide as solvent. However, the concentration of dimethylsulphoxide chosen to dissolve the test compounds was such that they did not show any antimicrobial property. The zone of inhibition was measured in millimeters.
Most of the tested compounds possessed significant antimicrobial activity when compared with that of gentamycin and amoxycillin. The test compound showing good qualitative antimicrobial property were further screened for their quantitative antimicrobial study by 96-well plate (Two fold dilution technique) using an ELISA Reader. It was found that coumarinoyl pyridinium salts 7a, 7c, 7d & 7i were found to be more active than that of other test compounds in those series as shown in table 1.
| Compds | R’ | R | Yield % | Antimicrobial activity* Zone of inhibition (mm) | ||||
|---|---|---|---|---|---|---|---|---|
| M.P. (ºC) | B.s. | E.c. | P.a. | S.a. | ||||
| 7a | 4–COOC2H5 | H | 85 | 210 | 28 | 14 | 26 | 30 |
| 7b | 4–COOC2H5 | Br | 75 | 228 | – | – | – | – |
| 7c | 4–COOC2H5 | Cl | 78 | 220 | 24 | 30 | 22 | 22 |
| 7d | 3–COOC2H5 | H | 75 | 224 | 28 | 26 | 28 | 26 |
| 7e | 3–COOC2H5 | Br | 79 | 225 | – | – | – | – |
| 7f | 3–COOC2H5 | Cl | 82 | 226 | – | – | – | – |
| 7g | 4–COOCH3 | H | 83 | 200 | – | 22 | – | – |
| 7h | 4–COOCH3 | Br | 78 | 208 | – | – | – | 26 |
| 7i | 4–COOCH3 | Cl | 82 | 218 | 24 | 24 | 22 | 32 |
| 7j | 3–COOCH3 | H | 79 | 218 | 26 | – | – | 22 |
| 7k | 3–COOCH3 | Br | 83 | 221 | 26 | – | – | 22 |
| 7l | 3–COOCH3 | Cl | 76 | 227 | – | 26 | 28 | 24 |
| 8a | – | H | 79 | 230 | – | – | – | – |
| 8b | – | Br | 76 | 219 | 20 | – | – | 29 |
| 8c | – | Cl | 75 | 220 | 20 | – | – | 24 |
| Standard | Amoxycillin | 35 | 33 | 34 | 39 | |||
| Gentamycin | 38 | 37 | 39 | 40 | ||||
Table 1: Physical Data and Antimicrobial Activity of Compounds 7a-l and 8a-c.
Acknowledgment
The authors are thankful to Prof. B.G. Shivananda, Principal, Al-Ameen College of Pharmacy, Bangalore for providing facilities.
References
- Nagaiah K, Krupadanam GLD, Srimannarayana G, Synthesis of angular furano Or pyrano coumarins from natural scopoletin. Indian J. Chem. 1998; 37B: 728-32.
- Venugopala KN, Jayashree BS, Mahesh Attimarad, Synthesis and evaluation of some substituted 2-arylamino coumarinyl thiazoles as potential NSAIDs. Asian J. of Chemistry. Asian J. Chem. 2004; 16(2): 872-74.
- Jayashree BS, Venugopala KN, Synthesis and characterization of schiff bases of 2’- amino-4’- (3-coumarinyl) thiazole as potential NSAIDs. Oriental J. Chem. 2004; 20(1): 123-26.
- Abramov MA, Dahaen WA, Novel Approach to Sulfide Derivatives of Furocoumarin Natural Products. Synthesis. 2000; 11: 1529-35.
- Kovac BN, 3-(Benzoxazol-2-yl)cromem-2 -one. Spectrochimica Acta. 2002; PartA, 58: 1483-87.
- Chilin A, Manzini P, Caffieri S, et al. Difurocoumarins: Psoralen analogs as photochemotherapeutic agents. J. Heterocyclic Chem. 2001; 38(2): 431-36.
- Monteiro FF, Leda Mathia, Ivo JC, et al. Prenylated Coumarins, Chalcone and New Cinnamic Acid and Dihydrocinnamic Acid Derivatives from Brosimum gaudichaudii. J. Braz. Chem. Soc. 2002; 13(2): 281-86.
- Hansch C, In; Comprehensive Medicinal Chemistry. New York: Pergamon Press Inc; 1990: 467.
- Ji M, Hu J, Hua W, et al. Synthesis and biological activity of substituted coumarines. Indian J .Chem. 2001; 40B: 1223-28.
- Suresh Khode, Veeresh Maddi, Prashant Aragade et al. Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eu. J. Med. Chem. 2009; 44: 1682-88.
- Kalkhambkar RG, Kulkarni GM, Shivkumar H, et al. Synthesis of novel triheterocyclic thiazoles as anti-inflammatory and analgesic agents, Eu. J. Med Chem. 2007; 42: 1272-79.
- Prescott LM, Harley JP, Klein DA, In; Medical Microbiology and Immunology, 2nd Edn: New York: McGraw-Hill; 1979: 105.