Structure Activity of CB1 Cannabinoid Receptor Antagonists
2 Department of Pharmacology, Institute of Biological Sciences, Federal University of de Minas Gerais, Belo Horizonte, Brazil
3 Faculty of Pharmacy, Federal University of Ouro Preto, Ouro Preto, Brazil
Citation: Costa ED, Garcia DCG, Correa CM. Structure Activity of CB1 Cannabinoid Receptor Antagonists. J Basic Clin Pharma 2018;9:53-62.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
The endocannabinoid system is an endogenous signaling system which acts in the regulation of energetic homeostasis and of the lipid and carbohydrate metabolism and in many another mechanism. CB1 receptor, one of the receptors involved in the endocannabinoid system, belongs to a superfamily of G-protein activated receptors, was shown to be an effective target to develop drugs with a therapeutic action on obesity rationally. Rimonabant is the first antagonist of this receptor drawn up to treat obesity. Furthermore, several other cannabinoid-1 receptor blockers have been planned to deal with this and other conditions such as schizophrenia, type II diabetes, alcoholism, smoking and Alzheimer’s disease. However, many studies have been demonstrating the most frequent spontaneous reports of depression and fatal and non-fatal behavior. Despite some beneficial properties of CB1 receptors antagonist, also some disadvantages may be considered and the scientific community still needs to have caution.
Keywords
Endocannabinoid system; CB-1 receptor; rimonabant; obesity; depression
ENDOCANNABINOID SYSTEM (ECS)
The use of Cannabis sativa (marijuana) for therapeutic and recreational purposes, has been described since 2.600 BC. The Chinese emperor Huangdi recommended its use for menstrual and articulation pains and abdominal cramps.[1] Based on this traditional knowledge, researchers have been able to identify and chemically characterize the main active compound of Cannabis sativa, ?9-tetrahydrocannabinol (?9THC)[2,3] Its effects in the organism involve both changes in memory and in cognition such as euphoria, analgesia, hypothermia and sedation.
The use of Cannabis for therapeutic purposes has resulted in the development of drugs (Nabilone and Marinol). The synthetic structure of ?9 THC has been authorized for use in special cases in Europe and United States. They have been used in capsules in the treatment of neoplasms to relieve nausea and vomiting caused by chemotherapy and in the treatment of patients with acquired immunodeficiency syndrome AIDS and with the purpose of preventing cachexia.[4-6] In the 1990s, several scientific advances allowed the endocannabinoid system to be more widely explained. CB1[7] and CB2 cannabinoid receptors have been cloned, and endogenous ligands (endocannabinoids) have been found to play a role in this system.[8,9]
ENDOGENOUS CANNABINOIDS
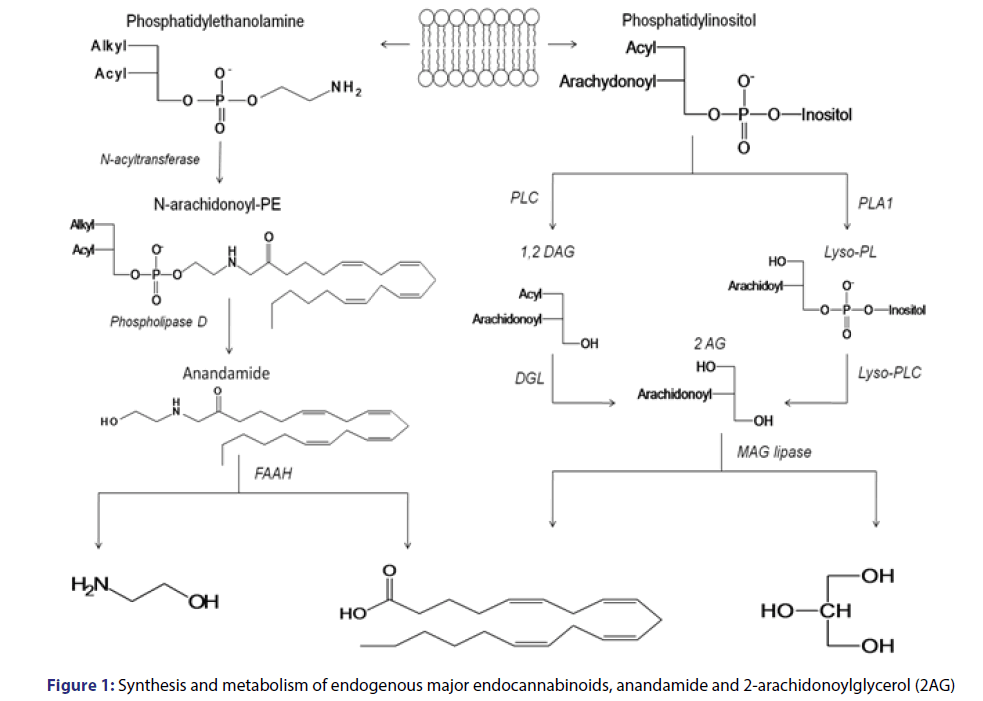
Endocannabinoids are substances derivates from long-chain polyunsaturated fatty acids, originated from their degradation by phospholipases which are activated by the calcium ion. There are several endogenous ligands for this system, the main ones are anandamide, an eicosanoid derivative initially identified from lipid extracts from swine brain[9] and 2-arachidonoyl-glycerol (2-AG), which presents a higher intrinsic activity than anandamide in both cannabinoid receptors. [10,11] On the body, 2-AG is present in higher concentration than anandamide. However, it has a short half-life, as it is rapidly degraded by the action of esterases.[12]
The anandamide synthesis [Figure 1] occurs after phosphatidylethanolamine cleavage, catalyzed by N-acyltransferase. It generates the N-arachidonoyl-phosphatidylethanolamine (N-arachinonyl-PE) which may be stored in the membrane and converted in anandamide by phospholipase D activated by Ca2+.[13,14]
As for 2-AG ligand, it seems that it has two important biosynthetic pathways [Figure 1] one which is degraded from phosphatidylinositol to 1,2-diacylglycerol (DAG); then catalyzed by phospholipase C (PLC); followed by the formation of 2-AG from DAG by diacylglycerol-lipase (DGL) catalysis. The second biochemical pathway which may originate 2-AG also occurs in two phases: catalyzed by phospholipase A1 (PLA1) originating 2-arachidonyl-lysophospholipid (Lyso-PL) from phosphatidyl inositol; and one which originates 2-AG from Lyso-PL by the action of Lyso-PLC. Since 2-AG is metabolized in presynaptic neurons it is degraded by the action of the enzymes monoacylglycerol lipase (MAGLipase) and FAAH, producing glycerol and arachidonic acid.
Anandamide is mainly metabolized by post-synaptic neurons and is degraded by the action of N-acylphosphatidylethanolamine (NAPEPL), selective phospholipase, producing arachidonic acid and ethanolamine. Endogenous cannabinoids are stored in lysosomal vesicles in nerve endings, due to their high lipophilic character. Their production and release occur ├ā┬ó├éŌé¼├é┼ōon demand├ā┬ó├éŌé¼├é┬Ø due to the physiological needs of the organism.[14-18] This system seems to act by modulating several physiological functions, acting in the CNS and also peripherally, on several sites, establishing a communication network between peripheral and central nervous systems.
Types of cannabinoid receptors
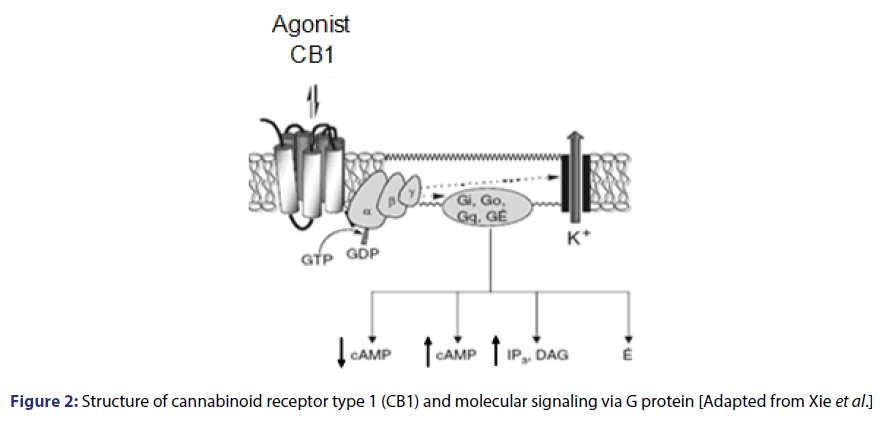
The mechanism of action of the endocannabinoid system involves two types of receptors, CB1 and CB2, which have been pharmacologically located and characterized. The CB1 receptor is more abundant in the CNS and is mainly expressed in presynaptic neurons and, in a smaller amount in astrocytes and oligodendrocytes.[19] It is also found in many other tissues, including liver and the pancreas.[20] adipocytes[21] gastrointestinal tract[22] and skeletal muscle.[23] CB2 receptors are located mainly in cells from the immunological system, but they may also be expressed in a limited way in the brain and other nonimmune tissues.[24] Including adipocytes.[25] Both receptors, share 44% of general homology despite differences in the expression profile and pharmacological standards.[26] Cannabinoid receptors have seven transmembrane domain, with an extracellular and an intracellular handle binding sites. They belong to the superfamily of metabotropic receptors, which are coupled to inhibitory G protein (Gi/o).[12,14]
CB receptors play an inhibitory neuromodulatory role acting as a retrograde messenger. After synthesizing postsynaptic neurons, endocannabinoids passively diffuse and may act on presynaptic receptors and block the Ca2+ entry. This cellular event may occur through the direct interaction of G protein ├āŲÆ├é┼Ė? subunits or indirectly by opening K+ channels with consequent hyperpolarization that result in the inhibition of fusion and the release of neurotransmitter vesicles. The activation of this receptor inhibits adenylate-cyclase thus closing calcium channels, opening potassium channels and stimulating kinase proteins [Figure 2].[14,27-30]
ENDOCANNABINOID SYSTEM AND EATING ATTITUDES
Researches in animal├ā┬ó├éŌé¼├éŌäós models have shown that the endocannabinoid system is involved with the regulation of eating behavior.[21] Both exogenous and endogenous cannabinoids tend to increase food intake in animals and humans.[31,32]
Due to this confirmation, we can consider that the block of CB1 receptors should affect eating attitudes. The first evidence of the effect of the compound SR146716 (rimonabant) on circuits which modulate appetite was based on observing how it inhibits sucrose, ethanol and food intake, without changing other attitudes.[33,34]
Posterior studies with this drug have shown that it reduces the intake of palatable foods in fed animals or there is a decreased food intake for a short time in animals with food restriction.[1] When administered for knockout mice for the CB1 receptor, rimonabant does not produce any effect, showing that its action in the regulation of appetite is related to the endocannabinoid pathway and its particular site of action.[35,36]
In a subsequent study Ravinet Trillou et al.[26] have shown too that CB1 knockout mice have been more resistant to gain weight on a lipid-rich diet for the same receptor. Jbilo et al.[37] Administered rimonabant or placebo in a model of animal obesity which is more like human obesity, and have found that the white adipose tissue and the brown adipose tissue were 64% and 46% smaller (p<0.001) in rimonabanttreated animals when compared to placebo. The difference in the mean diameter of adipocytes was 57% lower, and the fat storage capacity was estimated to be 90% lower in the group treated with this drug.
Also, rimonabant is able to increase the adiponectin expression in obese animals.[38] Adiponectin, a plasma protein which is exclusively expressed and secreted in the adipose tissue. The administration of adiponectin induces the oxidation of free fatty acids, reduces hyperglycemia and hyperinsulinemia and reduces body weight.[39,40]
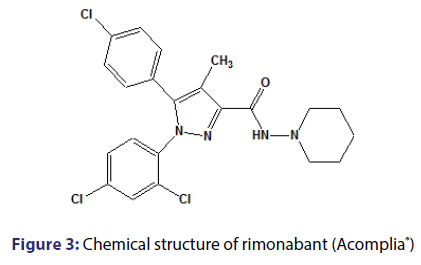
Rimonabant was showed to inhibit the proliferation and delays the maturation of preadipocytes in rats, in addition to reducing hepatic fatty acid synthesis.[20,41,42] With all the effects brought about weight loss through the endocannabinoid system, antagonism of type 1 cannabinoid receptors (CB1) has emerged as a highly promising strategy to treat obesity, metabolic disorders and other conditions related to smoking and alcohol.[43] Rimonabant [Figure 3], N-(piperidine-1-il)- 5-[4-chlorophenyl]-1-(2,4- dichlorophenyl)- 4-methyl-1H-pyrazole-3- carboxamide [Acomplia├āŌĆÜ├é┬«; Sanofi-Aventis], was the first antagonist of CB1 receptors to be found and well-characterized (CB1 Ki=2nM; CB2 Ki>1000nM).[44,45] It is the first member of the class of compounds which present pharmacological activity associated with the endocannabinoid system.
When orally ingested, this drug has been shown to produce a selective antagonist effect, presenting a prolonged therapeutic action and high affinity for receptors from the CNS, and a lower affinity for cannabinoid receptors found in the peripheral nervous system. Rimonabant has also been shown to cross the hematoencephalic barrier and exerce its mechanism of action both at the central and at the peripheral levels (Fremming). Despite the advantages for the obesity treatment, in the clinical trials performed, rimonabant has shown adverse effects associated to the CNS: depression, anxiety, dizziness and insomnia; and gastrointestinal effects: nausea and diarrhea.[46]
Rimonabant reversed agonist activity has also been shown both in in vivo and in vitro experiments. This drug has been suggested to act through three different mechanisms: competing with endogenous cannabinoids, allosterically modulating CB1 receptor activity or even by an independent CB1 receptor mechanism.[47]
In a study proposed by Hurst et al.[48] rimonabant reversed agonist activity is explained by the coexistence of two states of CB1 receptor: an active and an inactive one. The important interaction for this activity is performed with the Lys-192 amino acid with the receptor only when it is inactive. Recent studies also investigated the use of this drug to treat chronic smoking and alcoholism as well as schizophrenia (Clinical trials).
Relationship Between Structure and Activity Of Rimonbant and Taranabant
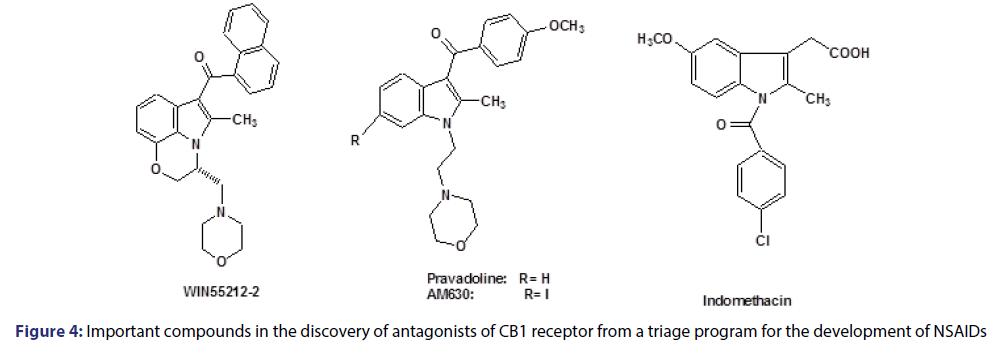
About 18 years ago, the class of indolic amine-alkylated (IAA) compounds was found by based on cannabinoid agonists, exemplified by the WIN-55212-2 compound [Figure 4], in a program which was performed to develop nonsteroidal anti-inflammatory drugs (NSAIDs), from indomethacin.[49]
These compounds were structurally related to pravadoline antiinflammatory drug.[50] Afterward, other synthetic efforts led to the discovery of CB1 receptor antagonist and partial agonists, such as AM630 (iodo-pravadoline), allowing us to conduct a study of the structure-activity relationship which developed compounds that are able to effectively block CB1 receptor such as rimonabant.[51]
Studies have confirmed that WIN-55212-2 compound has the same linking region in the CB1 receptor site as rimonabant, but presents a different site from that of endogenous agonist anandamide.[52] After discovering rimonabant in 1994, the planning of selective blockers of CB1 receptor started to represent a promising therapeutic strategy to treat obesity and other medical conditions related to the endocannabinoid system. Aiming to understand structural characteristics which control its selectivity, several studies have been carried out regarding the structure-activity relationship (SAR) and the quantitative relationships between the chemical structure and the biological activity (QSAR). These is fundamental to guide the synthesis of new molecules with optimized properties, minimizing the universe of compounds to be synthesized and considered in triage programs.
The structure-activity relationship of rimonabant is well-characterized and is based on three most important aspects: first, it├ā┬ó├éŌé¼├éŌäós the affinity to CB1 receptor, then its ability to block the same receptor, and finally, the analysis of the best pharmacological response (Nakamura-Palacios). Promising results from rimonabant and potential therapeutic application of CB1 receptor antagonists have stimulated the search for analogs with a similar pharmacological profile.[49] In 1999,[53] proposed a study to evaluate the structure-activity relationship of pyrazole derivatives as cannabinoid receptor antagonists. A series of pyrazole derivatives have been designed and synthesized to help to characterise cannabinoid receptors and also to serve as potentially useful pharmacological probes.
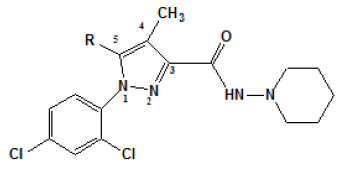
The studies about the affinity of these synthesized pyrazole compounds have evaluated anterior part of the brain (CB1) and, in rats├ā┬ó├éŌé¼├éŌäó spleen membranes (CB2). An assay was also applied to measure the specificity of these compounds by the displacement of the compound [3H] from cannabinoid sites. Using rimonabant (compound 1) as a baseline, initially, the substitution was performed at the position 5 from the pyrazole ring [Table 1]. In this early series, compound 7, para-iodinephenyl, was the one with more affinity (Ki value of 7.5 nM). It is interesting to observe that substitutions performed at para-position in 3, 5 and 7 derivatives had more affinity and selectivity for CB1 receptor than the respective substitution in the orto-position in 4, 6 and 8 derivatives. In addition, the affinity was reduced when the aromatic ring of compound 2 was substituted by an aliphatic group such as in the 10 compound.
| Compound | R | Ki (nM)a | |
|---|---|---|---|
| CB1 | CB2 | ||
| 1 | p-Cl-Ph | 11.50 | 16.4 |
| 2 | Ph | 123 | 217 |
| 3 | p-NO2-Ph | 57.5 | 252 |
| 4 | o-NO2-Ph | 255 | 691 |
| 5 | p-NH2-Ph | 81.5 | |
| 6 | o-NH2-Ph | 46.009 | |
| 7 | p-I-Ph | 7.49 | 2290 |
| 8 | o-I-Ph | 53.8057 | |
| 9 | p-Br-Ph | 16.8 | 1430 |
| 10 | Et | 183 | 744 |
Table 1: Ki. aValues of ligands for CB1 (anterior parts of rat├ā┬ó├éŌé¼├éŌäós brain) and CB2 (rat├ā┬ó├éŌé¼├éŌäós spleen) receptors
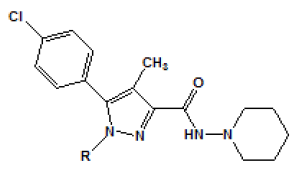
The substitution at position 1 of the pyrazole ring was also explored in the study of Lan [Table 2]. In this case, another substitute such as para-4-chlorophenyl derivative 11 led to a decreased affinity for CB1 receptor. The substitution of carboxamide at position 3 of the pyrazole ring has also been investigated. The N,N-Piperidinyl analog was also found to result in a better selectivity for CB1 receptor [Table 3].
| Compound | R | Ki (nM)a | |
|---|---|---|---|
| CB1 | CB2 | ||
| 1 | 2,4-di-Cl-Ph | 11.50 | 16.4 |
| 2 | 4-Cl- Ph | 60.4 | 836 |
Table 2: Modified ligands at position 1 of the pyrazole ring (Adapted from Lan et al.)
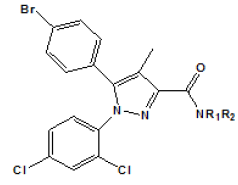
| Compound | R1 | R2 | Ki (nM)a | |
|---|---|---|---|---|
| CB1 | CB2 | |||
| 1 | H | Pyrrolidinyl | 17.10 | 1310 |
| 2 | H | Piperidinyl | 16.80 | 1430 |
| 3 | H | Homopiperidinyl | 7.85 | 215 |
| 4 | H | Morpholine-4 | 53.9 | 2450 |
| 5 | - | -(CH2)5- | 125 | 4580 |
| 6 | H | Cyclohexyl | 11.7 | 1010 |
| 7 | Me | Cyclohexyl | 76.7 | 1260 |
| 8 | H | 2-ethanol | 1120.00 | 1.9X104 |
| 9 | H | Phenyl | 31 | 6750 |
Table 3: Modified ligands at position 3 (Adapted from Lan et al.)
In a study proposed by Shim et al.[54] a comparative molecular field analysis (CoMFA), a 3D-QSAR method which is usually employed in the structural optimization process of leading compounds was used as a computational tool. 3-D models have been proposed for the molecule of rimonabant and its analogs based on observations of the affinity of these compounds (Ki) and on the displacement of radioligand [3H] CP55940.
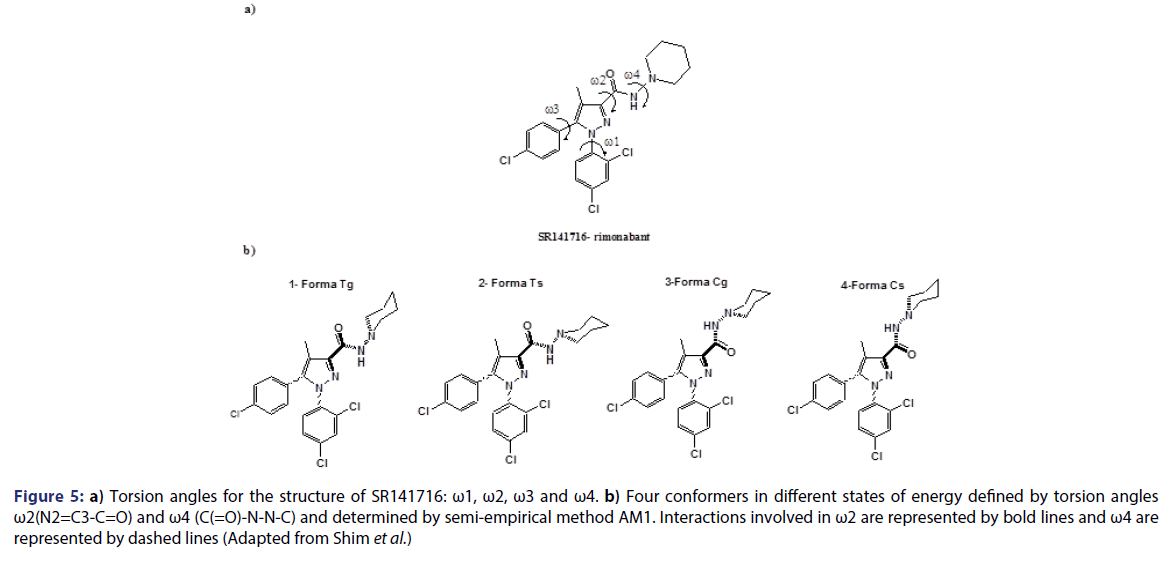
Six distinct models of CoMFA have been made and divided in two main groups: one in which conformers assume a deprotonated form; and another one in which conformers assume a protonated form. The conformational analysis of these compounds was performed using a semi-empirical method AM1. The conformations obtained have been classified in 4 different forms: Tg, Ts, Cg and Cs. The first letter T or C refers to s-trans or s-cis, respectively, associated to the torsion angle ?2. Whereas the second letter s or g refers to torsion angle refers, respectively, to torsion angles ?4 of +120├āŌĆÜ├é┬║ and -60├āŌĆÜ├é┬║ (gauche) [Figure 5].
Figure 5: a) Torsion angles for the structure of SR141716: ?1, ?2, ?3 and ?4. b) Four conformers in different states of energy defined by torsion angles ?2(N2=C3-C=O) and ?4 (C(=O)-N-N-C) and determined by semi-empirical method AM1. Interactions involved in ?2 are represented by bold lines and ?4 are represented by dashed lines (Adapted from Shim et al.)
For the deprotonated form, the most energetically stable conformation was Tg, with about 2, 5 and 7 Kcal/mol more stable than Cg, Ts and Cs. In the case of the protonated form, the most stable energetically conformation was Ts, with about 3, 4 and 7 Kcal/mol more stable than Tg, Cs and Cg. respectively. In each case, the best model was proposed based on the confirmation of each conformation of the group of 26 compounds being tested [Table 4]. N1 substitutes associated to high affinity are 2,4-dichlorophenyl (compound 1), n-pentyl (compound 33), n-hexyl (compound 35). These substitutes alkyl, may have a double conformation and, eventually, Interact with the same residues in the receptor as 2,4- dichlorophenyl. It is interesting to note that a limitation in the size of the hydrophobic cluster associated to C3 of the pyrazole ring has been seen for this class of compounds. Thus, we may assume that the groups linked to N1 and to C3 from rimonabant (SR141716), provide significant hydrophobic interactions with CB1 receptor.
| Compound | R1 | R2 | R3 | Ki (nM)a |
|---|---|---|---|---|
| 1(SR141716) | 2.4-dichlorophenyl | N-(piperidine-1-il)amide | Cl | 1.3 |
| 2 | 2, 4-dichlorophenyl | N-(piperidine-1-il)amide | I | 6 |
| 3 | 4-chlorophenyl | N-(piperidine-1-il)amide | Cl | 55 |
| 4 | 4-nitrophenyl | N-(piperidine-1-il)amide | Cl | 109 |
| 5 | 4-aminophenyl | N-(piperidine-1-il)amide | Cl | 72 |
| 6 | 2,4-dichlorophenyl | N-(cyclohexyl)amide | Br | 15 |
| 7 | 2,4-dichlorophenyl | N-(hydroxietyl)amide | Br | 56 |
| 8 | 2,4-dichlorophenyl | N-(morfolin-4-il)amide | Br | 165 |
| 9 | 2,4-dichlorophenyl | N-(morfolin-4-il)amide | Br | 19 |
| 10 | Cyclohexyl | N-(piperidine-1-il)amide | H | 391 |
| 11 | n-propyl | N-(piperidine-1-il)amide | H | 771 |
| 12 | n-butyl | N-(piperidine-1-il)amide | H | 187 |
| 13 | n-pentyl | N-(piperidine-1-il)amide | H | 23 |
| 14 | n-pentyl | N-(piperidine-1-il)amide | Br | 63 |
| 15 | n-hexyl | N-(piperidine-1-il)amide | H | 21 |
| 16 | n-heptyl | N-(piperidine-1-il)amide | H | 47 |
| 17 | 2,4-dichlorophenyl | (piperidine-1-il)ethoxymethyl | Cl | 232 |
| 18 | 2,4-dichlorophenyl | (cyclohexyl)methoxymethyl | Cl | 100 |
| 19 | 2,4-dichlorophenyl | 4-fluorobenzyloxymethyl | Cl | 6 |
| 20 | 2,4-dichlorophenyl | N-n-pentyl-amide | Cl | 3 |
| 21 | 2,4-dichlorophenyl | N-n-heptyl-amide | Cl | 3 |
| 22 | 2,4-dichlorophenyl | Pentylcarnonyl | Cl | 25 |
| 23 | 4-sec-butylphenyl | N-(piperidine-1-il)amide | Cl | 37 |
| 24 | 4-n-butylphenyl | N-(piperidine-1-il)amide | Cl | 256 |
| 25 | 4-n-pentylphenyl | N-(piperidine-1-il)amide | Cl | 1360 |
| 26 | 2.4-dichlorophenyl | N-(piperidine-1-il)amide | 1 |
Table 4: Affinities of rimonabant and its analogues such as the CB1 receptor (Adapted from Shim et al.)
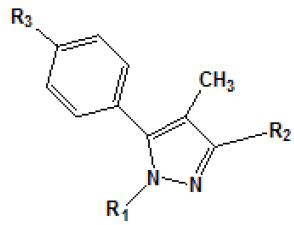
The analysis of electrostatic fields by CoMFA has shown that dipoledipole interactions and hydrogen bonding interaction exerted by the carboxamide group with the cannabinoid receptor are important to explain the variation in affinity between these compounds and the receptor. Functional groups with polar characteristics, such as oxygen from the carboxamide group, nitrogen from the piperidine ring, and other oxygen and nitrogen atoms (such as in compounds 28, 29 and 37-39 [Table 4]) present a possible substitute of C3 in the pyrazole ring, seem to be important for electrostatic interactions with the receptor. The analysis has also revealed that steric contributions in the interaction with the receptor are substantially more significant than electrostatic interactions with the target site, and that the region around the dichlorophenyl group bound to N1 is particularly important for these steric interactions.
It has also been suggested that the amide substitute present in C3 is important not only to prevent the agonist activity, but also to induce or stabilize the receptor in a necessary conformation so that a reverse agonist activity occurs. Another study proposed by Chen et al. in [55] researched the main structural requirements for the selective and blocking activity of arylpyrazoles in CB1 and CB2 receptors. Chen├ā┬ó├éŌé¼├éŌäós study has shown that the variation of affinity to the binding character of arylpyrazolesis dominated by steric interactions in the cannabinoid receptors. The result is coherent with the well-known importance of hydrophobic interactions of classical cannabinoids for the activity.
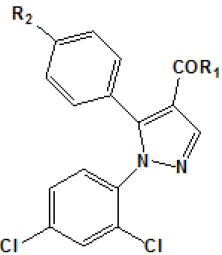
In another work, Menozzi et al.[56] proposed a study of the structureactivity relationship of a series of compounds presenting structural variations in the molecule of rimonabant. Thus, a model of threedimensional homology of CB1 receptor was built with molecular modeling techniques, from the structure of rhodopsin protein. T??he aminoacid sequence of the CB1 receptor was alignedon the basis of amino acid residues on the basis of amino acid residues highly conserved. Later, rimonabant (molecule that served as model) was docked at the putative site of the CB1 receptor by the tool MOE. The compounds [3H]-CP 55940 (0.5 nM) and [3H]-WIN 55212-2 (0.8 nM) e [3H]-WIN 55212-2 (0.8nM) were used as radio ligands in this experiment to mark both the CB1 receptor as CB2.
Results obtained are shown in Table 5 and expressed based on the percentage of radio ligand displacement by synthesized derivatives. In biological trials, virtually all compounds tested have shown a significant competitive bonding (radio ligand displacement >50% to 10 ├āŌĆÜ├é┬ĄM), but a low selectivity for CB1 receptor. In this study, the compound 47 was the one which presented an interaction which was more like rimonabant, presenting a competitive bonding of 79% for CB1 receptor and 37% for CB2 receptor, respectively.
| Compound | R1 | R2 | Radioligand displacement [%] at 10 ├āŌĆÜ├é┬ĄM | |
|---|---|---|---|---|
| CB1 Receptor | CB2 Receptor | |||
| 1 | 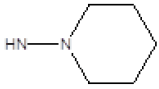 |
Cl | 79 | 37 |
| 2 | 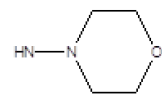 |
Cl | 65 | 12 |
| 3 | 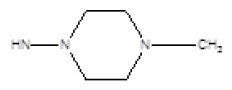 |
Cl | 61 | -23 |
| 4 | 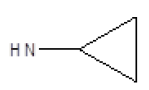 |
Cl | 87 | 30 |
| 5 | 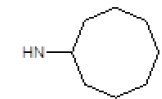 |
Cl | 84 | 44 |
| 6 | 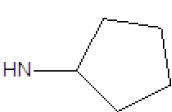 |
Cl | 82 | 61 |
| 7 | 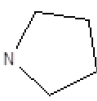 |
Cl | 49 | 43 |
| 8 | 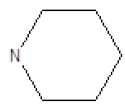 |
Cl | 59 | 45 |
| 9 | 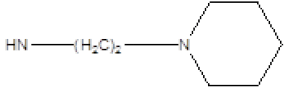 |
Cl | 39 | 14 |
| 10 | 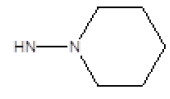 |
F | 43 | 7 |
| 11 | 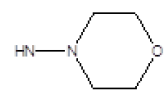 |
F | 30 | -9 |
| 12 | 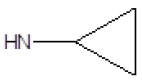 |
F | 62 | 7 |
| 12 | 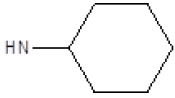 |
CH3 | 48 | 46 |
| 13 | 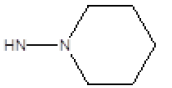 |
CH3 | 29 | 27 |
| 14 | 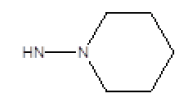 |
OCH3 | 58 | 70 |
| 15 | 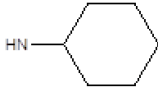 |
OCH3 | 64 | 76 |
| 16 | 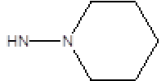 |
Br | 74 | 55 |
| 17 | 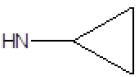 |
Br | 84 | 43 |
| 18 | 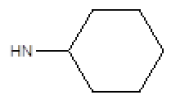 |
I | 94 | 84 |
| 19 |  |
I | 91 | 60 |
Table 5: Relative affinities of rimonabant and its analogues such as CB-1 receptor
N-cycloallyls (compounds 50-52) have shown similar properties or had their affinity slightly increased when compared to compound 47 and with other hydrazides (compounds48 and 49). N,N-disubstitution of carboxamide function (compounds 53 and 54) resulted in a decreased affinity and selectivity for CB1 receptor. The insertion of an ethylene between the 1-piperidinyl group and the nitrogen atom from the carboxamide group, such as the compound 55 caused a decreased affinity of both receptors: CB1 and CB2.
Substitutions of chlorine atom in the para-5-phenyl ring by fluorine (compounds 56-58) or by the methyl group (compounds 59 and 60) present a decreased affinity for both cannabinoid receptors, while 61 and 62 derivatives have shown an increased affinity and selectivity for CB2 receptors. In general, para-substitute compounds 63-66 synthesized with bromine and iodine in the chemical structure have shown a slightly higher affinity than their corresponding chlorinated analogues in both receptors.
As evaluated by computational studies, all the rimonabant derivatives suggested by the study of Menozzi et al. share the idea of hydrogen bonding with Lys192 amino acid, comparable to that of rimonabant. The two aromatic rings present in the molecule are involved in interactions of p-p stacking with Trp279, Trp356 and Phe379.
In contrast with rimonabant, compound 47 does not interact with Phe174, Val196, Met384 and Leu387. The loss of this Anchorage of CB1 receptor may indicate less affinity of pyrazole derivatives suggested in this study when compared to rimonabant. Thus, according to data presented in the study, the hydrogen bonding with Lys192 residue seems to be essential for a good positioning of the ligand inside the receptor. However, this interaction does not seem to be enough to change the Constant inhibition of CB1 receptor antagonists by values which provide a considerable affinity. The result of this study has also suggested the need of a balance between hydrophobic and hydrophilic substitutes present in the molecule for a better interaction with the receptor.
Study On The Interactions Between Rimonabant And Cb1 Receptor By Means Of Molecular Modeling
From the 3D homology model of CB1 receptor, based on the bovine rhodopsin structure and using more elaborated computational approaches, it was possible to obtain information on key interactions between rimonabant and amino acid residues of the CB1 receptor.[57] In studies proposed by Montero et al.[58] multiple sequences of alignment have shown that CB1 receptor presented 21% of visual identity with rhodopsin. After aligning this protein with cannabinoid CB1 receptor, the main residues were shown to be preserved and the main differences were in the transmembrane region of TM5.
Rhodopsin has a highly preserved Pro215 amino acid which is not present in the CB1 receptor sequence and, in addition, two tyrosine residues are present in the CB1 receptor in a region in which rhodopsin has a single tyrosine. Another difference is absent in CB1 of cysteine residues present in the rhodopsin structure. A model of association between CB1 receptor and rimonabant has been proposed and, the key interaction was verified to involve the hydrogen bonding between carbonyl group and amino acid Lys192 of CB1 receptor [Figure 6]. This bonding plays a stabilizing role in the residues Lys192-Asp366 (salt bridge) of intracellular ends of transmembrane helices 3 and 6.[57] This particular salt bridge is induced by a pronounced torsion in Pro358 in the transmembrane helix 6 which is in the inactive state of the receptor, is absent in the active receptor.[57]
Associations between CB1 receptor and rimonabant are strengthened by interactions between the aromatic ring of 2,4-dichlorophenyl and Trp279/Phe200/Trp356 residues and by interactions among Tyr275/ Trp255/Phe278 residues in the other para-chlorophenyl ring which is linked to the pyrazole ring. Another important interaction in the CB1 receptor well is performed between residues of Val196/Phe170/ Leu387/ Met384 amino acids and the group with a strong lipophilic feature, in the case of rimonabant, the piperidinyl group.[57]
Conclusion And Perspectives
Since the characterization of the first cannabinoid receptor in 1990, until the Discovery of the first effective blocker by means of signaling, a lot of advances have been seen in the development of compounds which may act as a new therapeutic strategy in obesity and in other related diseases. The effects of rimonabant have been tested in 42 studies, according to what was determined in a research in the Clinical Trials database, in several cases: such as atherogenic, dyslipidemic, in cardiovascular problems, type II diabetes and in alcoholism and smoking.
These underway researches are based on recent evidences, which show that the CB1 receptor stimulation reinforces lipogenesis, inhibits glucose and fatty acid oxidation acting in adipocytes, hepatocytes, pancreas and skeletal musculature. In addition, a stimulation of the endocannabinoid activity in the visceral adipose tissue, in the liver and in the pancreas, may play a role in the glucose intolerance and directly in the dyslipidemia and, therefore, regardless the indirect effects on food intake or weight.[20,59]
However, also there are some studies on the literature about the adverse effects of rimonabant. How already described, it can decrease the neurotransmitters release and the pacient may present depression, anxiety, dizziness and insomnia; and gastrointestinal effects: nausea and diarrhea (Majumdar). The most dangerous effect associated with rimonabant is the suicide. According to Thomas et al.[60] between 1998 and 2011 was identified most frequent spontaneous reports of depression, and fatal and non-fatal behavior on United Kingdom. Thus, we may conclude that, despite some beneficial properties of CB1 receptors antagonist, also there are some disadvantages that may be considered and that the scientific community still need to have caution with these drugs.[61-63]
Financial support and sponsorship
Nil.
Conflict of Interest
There are no conflicts of interest.
REFERENCES
- Arnone M. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacolo (Berl)1997;132:104-6.
- Avraham Y. Effects of the endocannabinoid noladin ether on body weight, food consumption, locomotor activity, and cognitive index in mice. Brain Res Bull 2005;65:17-23.
- Barth F, rinaldi carmona M. The development of cannabinoid antagonists. Curr Med Chem 1999;6:745-55.
- Beal JE. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 1995;10:89-97.
- Bensaid M. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003;63:908-14.
- Bisogno T. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J 1997;322:671-7.
- Bouaboula M. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem 1993;214:173-80.
- Chen JZ. 3D-QSAR studies of arylpyrazole antagonists of cannabinoid receptor subtypes CB1 and CB2. A combined NMR and CoMFA approach. J Med Chem 2006;49:625-36.
- Clinicaltrials.gov database. Accessed: September 3, 2016.
- Colombo G. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacolo (Berl) 2002;159:181-7.
- Combs TP. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 2001;108:1875-81.
- Cota D. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 2003;112:423-31.
- Devane WA. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988;34:605-13.
- Devane WA. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992;258:1946-9.
- Di marzo V, Bifulco M, De petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004;3:771-84.
- Di marzo V. Potential biosynthetic connections between the two cannabimimetic eicosanoids, anandamide and 2-arachidonoyl-glycerol, in mouse neuroblastoma cells. Biochem Biophys Res Commun 1996;227:281-8.
- Di marzo V. Fontana A. Anandamide, an endogenous cannabinomimetic eicosanoid 'killing two birds with one stone'. Prostaglandins Leukot Essent Fatty Acids 1995;53:1-11.
- Di marzo V. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994;372:686-91.
- Di marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci 2005;8:585-9.
- Fremming AB, Boyd TS. A Review of Rimonabanto A Novel Cannabinoid Type CB1 Receptor Antagonist. European J Clin Res Klinik und Forschung 2006:13-6.
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 2003;83:1017-66.
- Fride E. Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: evidence for a "CB3" receptor. Eur J Pharmacol 2003;461:27-34.
- Gary bobo M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol 2006;69:471-8.
- Gonsiorek W. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol 2000;57:1045-50.
- Haney M. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr 2007;45:545-54.
- Herkenham M. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991;11:563-83.
- Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol 1995;35:607-34.
- Howlett AC. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 2002;54:161-202.
- Huffman JW. Cannabimimetic indoles, pyrroles and indenes. Curr Med Chem 1999;6:705-20.
- Hurst DP. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol Pharmacol 2002;62:1274-87.
- Izzo AA. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol 2001;134:563-70.
- Jbilo O. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 2005;19:1567-9.
- Kirkham TC, Williams CM. Endogenous cannabinoids and appetite. Nutr Res Rev 2001;14:65-86.
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol 2002;12:324-30.
- Lan R. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem 1999;42:769-76.
- Lange JH. Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists. J Med Chem 2004;47:627-43.
- Lange JH, Kruse CG. Keynote review: Medicinal chemistry strategies to CB1 cannabinoid receptor antagonists. Drug Discov Today2005;10:693-702.
- Lange JH. Bioisosteric replacements of the pyrazole moiety of rimonabant: synthesis, biological properties, and molecular modeling investigations of thiazoles, triazoles, and imidazoles as potent and selective CB1 cannabinoid receptor antagonists. J Med Chem 2005;48:1823-38.
- Liu YL. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 2005;29:183-7.
- Matsuda LA. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990;346:561-4.
- Mcallister SD. An aromatic microdomain at the cannabinoid CB(1) receptor constitutes an agonist/inverse agonist binding region. J Med Chem 2003;46:5139-52.
- Menozzi G. Rational design, synthesis and biological evaluation of new 1,5-diarylpyrazole derivatives as CB1 receptor antagonists, structurally related to rimonabant. Eur J Med Chem 2008;43:2627-38.
- Micale V, Mazzola C, Drago F. Endocannabinoids and neurodegenerative diseases. Pharmacol Res 2007;56:382-92.
- Montero C. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur J Med Chem 2005;40:75-83.
- Nakamura Palacios EM, Moerschbaecher JM, Barker LA. The pharmacology of SR 141716A: A review. CNS Drug Rev 1999;5:43-58.
- Osei-hyiaman D. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005;115:1298-305.
- Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 2007;369:71-7.
- Pagano C. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 2007;92:4810-9.
- Pagotto U, Vicennati V, Pasquali R. The endocannabinoid system and the treatment of obesity. Ann Med 2005;37:270-5.
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 2005;76:1307-24.
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003;4:873-84.
- Ravinet Trillou C. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 2003;284:R345-53.
- Ravinet Trillou C. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 2004;28:640-8.
- Rinaldi-carmona M. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci 1995;56:1941-7.
- Rinaldi-carmona M. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 1994;350:240-4.
- Roche R. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol 2006;126:177-87.
- Savinainen JR. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br J Pharmacol 2001;134;664-72.
- Shim JY. Molecular interaction of the antagonist N-(piperidin-1-yl)-5-(4-chlorophenyl)-1- (2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide with the CB1 cannabinoid receptor. J Med Chem 2002;45:1447-59.
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 1997;388:773-8.
- Thomas G. Studies on the mechanism of spasmolytic activity of (O-methyl-)-N-(2,6-dihydroxybenzoyl)tyramine, a constituent of Aniba riparia (Nees) Mez. (Lauraceae), in rat uterus, rabbit aorta and guinea-pig alveolar leucocytes. J Pharm Pharmacol 1994;46:103-7.
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science 2002;296:678-82.
- Xie S. The endocannabinoid system and rimonabant: a new drug with a novel mechanism of action involving cannabinoid CB1 receptor antagonism--or inverse agonism--as potential obesity treatment and other therapeutic use. J Clin Pharm Ther 2007;32:209-31.
- Yamauchi T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941-6.