Stability-indicating RP-HPLC method for determination of tamsulosin HCL in pharmaceutical dosage form
- *Corresponding Author:
- G S Kumar
Department of Pharmaceutical Analysis & Quality Assurance, GITAM Institute of Pharmacy, GITAM University, Visakhapatnam - 530045, India.
E-mail: drgshivkumar@gitam.edu
Date of Received: 20-03-2012
Date of Accepted: 02-05-2012
Available Online: 15-05-2012
Abstract
A selective, specific and sensitive stability-indicating high-performance liquid chromatographic method was developed and validated for the determination of Tamsulosin in in pharmaceutical dosage forms. Celecoxib was used as Internal Standard (IS). The chromatographic conditions comprised of a reversed-phase Lichrocart / Lichrosphere C18 column (250 x 4.0 mm packed with 5 μ) with mobile phase consisting of a mixture of Acetonitrile: T.D.W. in the ratio (40: 60). Flow rate was 0.8 mL / min. Detection was carried out at 275 nm. The retention time of Tamsulosin HCl and Celecoxib were found to be 1.608 and 2.767min respectively and the linear regression analysis data for the calibration plots showed good linear relationship in the concentration range 1 - 200 μg/mL. The value of correlation coefficient, slope and intercept were, 0.9995, 0.7453 and 0.4584, respectively. Tamsulosin HCl was subjected to stress conditions of degradation in aqueous solutions including acidic, alkaline, oxidation, photolysis and thermal degradation. The developed method was validated with regard to linearity, accuracy, precision, selectivity and robustness and the method was found to be precise, accurate, linear and specific. The method was employed successfully for identification and determination of Tamsulosin in pharmaceutical preparations.
Keywords
Tamsulosin HCl, stability-indicating, HPLC, Validation.
Introduction
Tamsulosin HCl is a Uroselective α1A/ α1D blocker (α1A : α1B affinity 7-38 fold), chemically compound is 5-[2-[2-(2-ethoxyphenoxy) ethylamino]propyl]-2- methoxy-benzene sulfonamide Figure 1. is used in the improvment of BHP symptoms. Actions are (a) Selective α1A/ α1D blocker act on bladder base and prostate gland. (b) causes relaxation of smooth muscles in the bladder neck and prostate. Approximately 70% of the alpha1-receptors in human prostate are of alpha-1A subtype [3].
The International Conference on Harmonization (ICH) guideline entitled ‘Stability Testing of New Drug Substances and Products’ requires the stress testing to be carried out to elucidate the inherent stability characteristics of the active substance. Susceptibility to oxidation is one of the required tests (ICH, 1993, 1996). The hydrolytic and the photolytic stability are also required. An ideal stability-indicating method is one that quantifies the drug and also resolves its degradation products. Very few methods are reported in the literature regarding the clinical studies and no stability indicating method is available in the official compendia using HPLC for analysing Tamsulosin HCl in dosage forms.
Most of the analytical techniques for tamsulosin hydrochloride described in the literature are based on the various separation techniques like Spectrophotometric estimation [4-6], UV spectroscopy [7] in bulk and pharmaceutical dosage forms. Determination of the enantiomers of tamsulosin hydrochloride and its synthetic intermediates by chiral liquid chromatography [8],Stability-Indicating HPTLC methods in Bulk and Pharmaceutical Dosage Forms [9-10] , liquid chromatography methods in human plasma [11],in bulk drugs [12-14],liquid chromatography– mass spectrometry methods in dog [15],human plasma [16-20], in bulk drugs and formulations [21].
The aim of the present work was to develop an accurate, specific, reproducible, and stability indicating method for the determination of Tamsulosin HCl in the presence of its degradation products and related impurities as per ICH guideline.
Materials and Methods
Reagents and Materials
Bulk sample of Tamsulosin HCl was obtained from Sun Pharm lab,India and tablets (Label Claim: 10 mg per tablet, Product Name: Veltam and Manufacturer: Intas (Arron) was procured from the market. Triple distilled water (T.D.W) was used throughout the work. Methanol and Acetonitrile (HPLC grade) were procured from Merck chemicals
Chromatographic condition
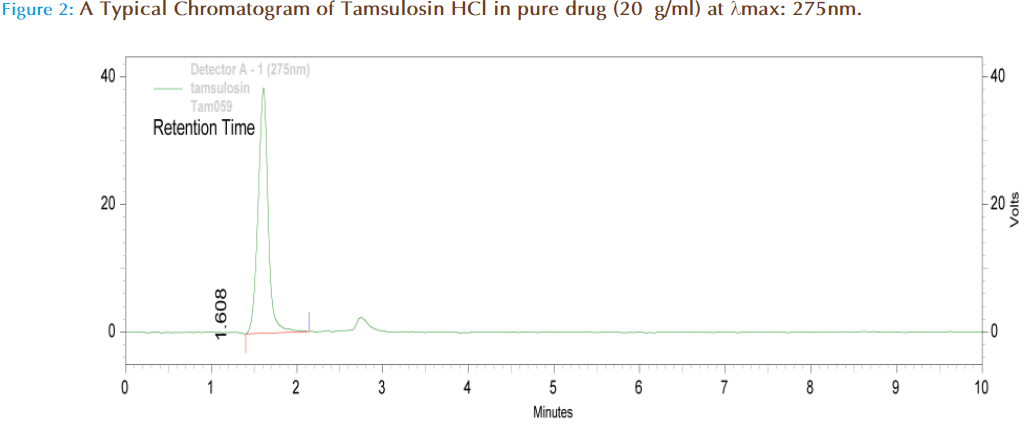
A Shimadzu HPLC separation module LC 10AT VP series pumps equipped with SPD 10AVP UV-Visible detector was used . Data acquisition was performed by Shimadzu Class-VP version 6.12 SPI software. Analysis was carried at 275nm with a Lichrocart / Lichrosphere 100 C-18 (0.4cm X 25 cm, packed with 5 μ) at ambient temperature (25ºC). The mobile phase was of a mixture of Acetonitrile: T.D.W. in the ratio 40: 60v/v. The flow rate was 0.8 ml / min and the retention time of Tamsulosin HCl and Celecoxib were found to be 1.608 and 2.767min respectively. The mobile phase was degassed and filtered through 0.45μm membrane filter before pumping into the HPLC system.
Preparation of standard stock solutions:
Stock solution of Tamsulosin HCl (1000 μg/ml) was prepared by dissolving 25 mg of Tamsulosin HCl in 25 ml of volumetric flask containing 10 ml of mobile phase. The solution was sonicated for about 20 minutes and then made up to volume with mobile phase. Working working standard solutions of Tamsulosin HCl was prepared by suitable dilution of the stock solution with appropriate mobile phase. Similarly stock solution of internal standard was prepared by dissolving 25 mg of Celecoxib in 10 ml of mobile phase, sonicated for 20min. then made up to the volume with mobile phase. Working standard solutions of Tamsulosin HCl were prepared by taking suitable aliquots of drug solution from the standard stock solution 1000 μg/ml, spiked with internal standard solution 0.1ml (From 1000 μg/ml stock of Celecoxib) and the volume was made upto 10 ml with mobile phase.
Preparation of sample solution
Twenty five tablets were weighed, finely powdered and an accurately weighed sample of powdered tablets equivalent to 25 mg of Tamsulosin HCl was extracted with mobile phase in a 25ml volumetric flask using ultra sonicator. This solution was filtered through 0.45μm filter paper. The solution obtained was diluted with the mobile phase so as to obtain a concentration in the range of linearity previously determined. An aliquot of the internal standard was added to the sample solution prior to the dilution. All determinations were carried out in triplicate.
Procedure for calibration curve
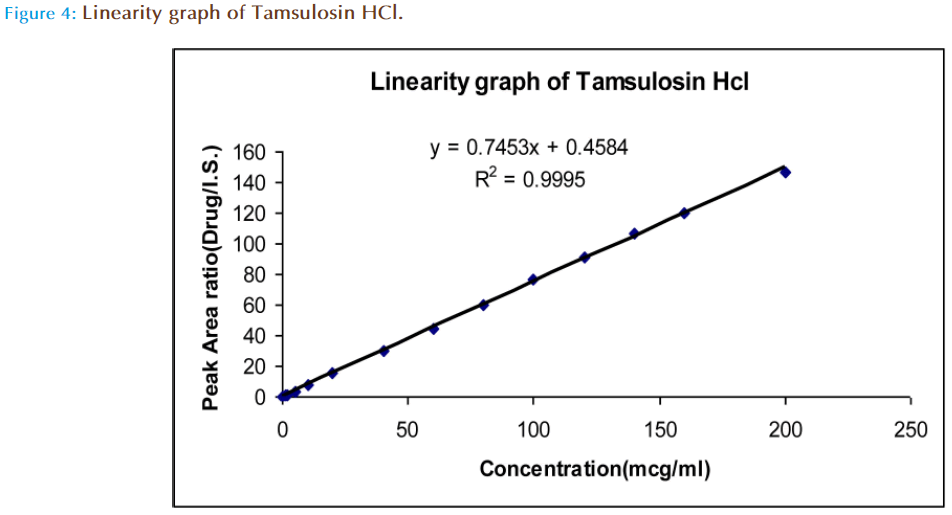
The contents of the mobile phase were filtered before use through 0.45μm filter paper, and pumped from the respective solvent reservoirs to the column at a specified flow rate. Prior to injection of the drug solutions, the column was equilibrated for at least 30 min with the mobile phase flowing through the systems. Then, 20 μl of each of standard and sample solutions were injected into the HPLC system for six times to get the chromatograms. The retention time, average peak areas and peak area ratios of drug to internal standard were recorded. Taking conc. plotted a graph on X-axis and peak area ratios on Y-axis. The linearity range was found to be in between 1-200 μg/ml for Tamsulosin HCl.
Method validation
Precision
The precision of each method was ascertained separately from the peak area ratios obtained by actual determination of eight replicates of a fixed amount of drug and internal standard. The percent relative standard deviations were calculated for Tamsulosin HCl and presented in the table 3.7. The precision of the assay was also determined in terms of intra day variation in the peak areas for a set of drug solutions and was calculated in terms of % RSD.
| Parameters | Values | |
|---|---|---|
| LOD | 0.36 | |
| LOQ | 0.74 | |
| Accuracy (%) | 98.88-100.03 | |
| Precision(%RSD) | ||
| Intraday(n=3) | 0.098 | |
| Interday(n=3) | 0.125 | |
| Ruggedness(%assay) | ||
| Analyst 1 | 99.34 | |
| Analyst 2 | 99.15 | |
| Robustness(%RSD) | Less than 2% | |
Table 1: Summary of Validation Parameters.
| Formulation | Labeled amount (mg) | Observedamount*(mg) | % Recovery by proposedmethod | %RSD |
|---|---|---|---|---|
| VELTAM INTAS (Arron) | 0.4 | 0.377 | 97.74±0.236 | 0.239 |
*Each value is average of five determinations ± standard deviation.
Table 2: Estimation of amount present in tablet dosage form.
| Stress Conditions | Auc | Retention Time | Drug Recovered (%) | Drug Decomposed (%) |
|---|---|---|---|---|
| Standard Drug | 92007 | 1.683 | 100 | 0 |
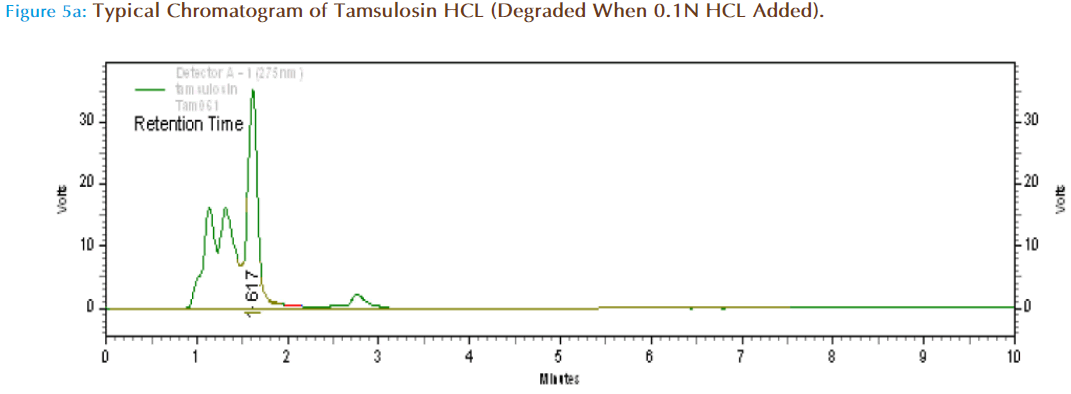
| Acidic Hydrolysis | 58776 | 1.617 | 63.88 | 36.12 |
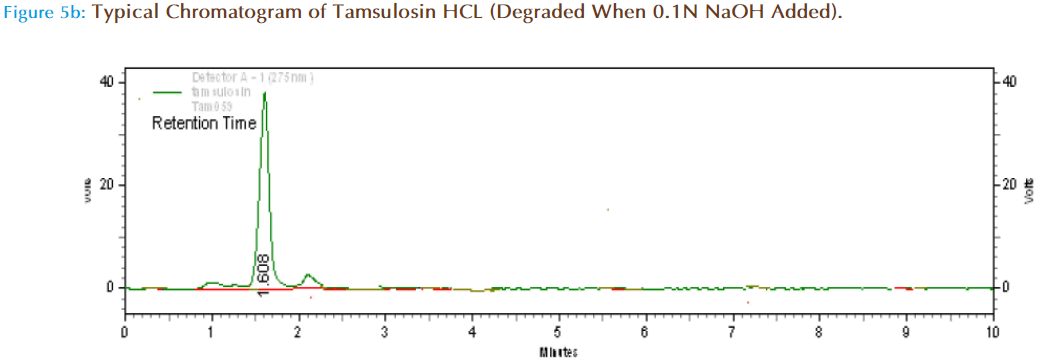
| Alkaline Hydrolysis | 61149 | 1.608 | 66.46 | 33.53 |
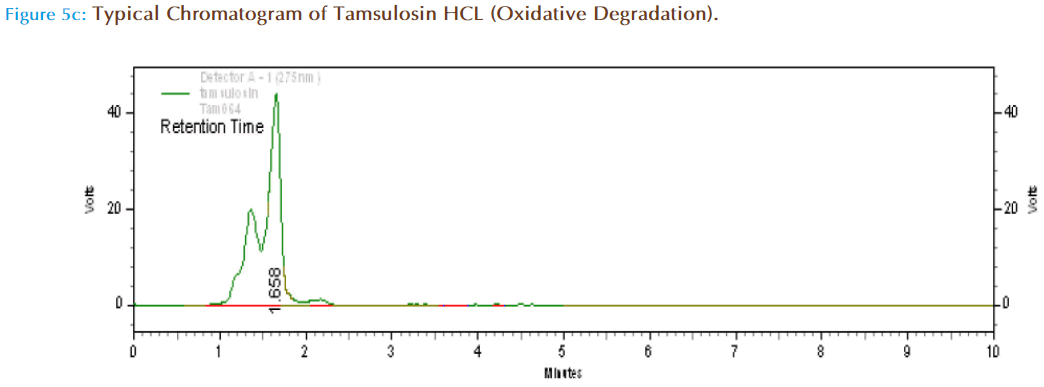
| Oxidative degradation | 78240 | 1.658 | 85.03 | 14.92 |
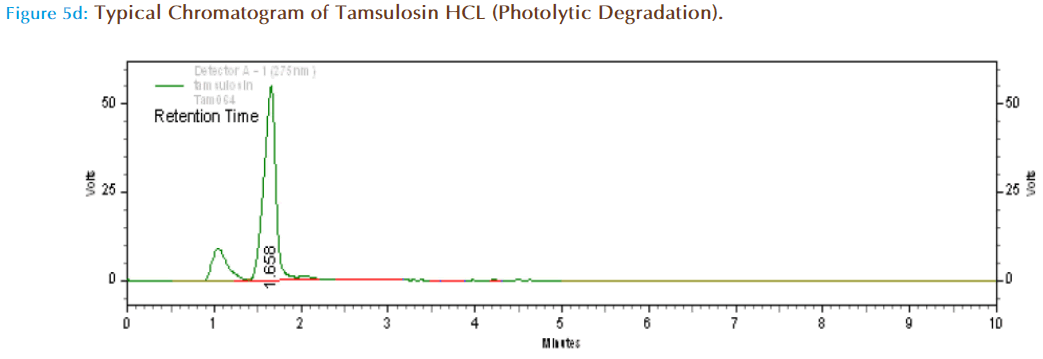
| Photolytic degradation | 82240 | 1.658 | 89.38 | 10.61 |
Table 3: Forced Degradation Readings.
Accuracy
To determine the accuracy of the proposed method, recovery studies were carried out by adding different amounts (80%, 100%, 120%) of bulk samples of BUP along with internal standard within the linearity ranges were taken and added to the pre-analyzed formulation of concentration 10 μg/ml. From that percentage recovery values were calculated.
Ruggedness and robustness of the method
Method robustness and ruggedness was determined by analyzing same sample at normal operating conditions and also by changing some operating analytical conditions such as column make, mobile phase composition, flow rate, instrument and analyst. The robustness and ruggedness of the method was established as the % deviation from mean assay value obtain from precision study is less than ±2.0%. LOD and LOQ were established by slope method as mentioned below.

Analysis of formulations
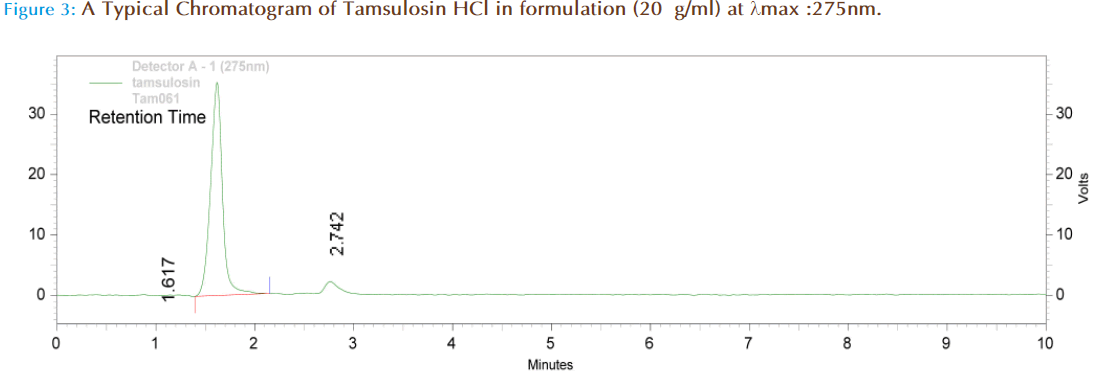
For analysis of commercial formulations, 25 tablets containing Tamsulosin HCl were taken and powdered. The powder equivalent to 10 mg of Tamsulosin HCl was taken in a 100 ml volumetric flask, containing 70 ml of mobile phase and sonicated for 30 minutes. The volume was made up to 100 ml with mobile phase and filtered to get a solution of concentrations 100 μg/ml. This was further diluted with to get a concentration within the linearity range. The amount of drug present in pharmaceutical formulation was calculated through peak area ratio of drug to that of internal standard by using the standard calibration curve (concentration in μg/ml was taken on X-axis and peak area ratio on Y-axis).
Specificity (Forced Degradation) Studies
The specificity of the method was demonstrated through forced degradation studies conducted on the sample using acid, alkaline, oxidative and photolytic degradations. The sample was exposed to these conditions and the main peak was studied for the peak purity, thus indicating that the method effectively separated the degradation products from the pure active ingredient.
1. Acid Degradation
About 10mg of Tamsulosin HCl pure drug was accurately weighed and transferred to 10ml volumetric flask and one ml of 0.1N HCl was added and kept aside for one hr and made up to volume with mobile phase. Then from this 10mcg/ml solution was prepared and injected in HPLC system to obtain chromatograms.
2. Alkaline Degradation
About 10mg of Tansulosin HCl pure drug was accurately weighed and transferred to 10ml volumetric flask and one ml of 0.1NNaOH was added and kept aside for one hr and made up to volume with mobile phase. Then from this 10mcg/ml solution was prepared and injected in HPLC system to obtain chromatograms.
3. Oxidative degradation
About 10mg of Tamsulosin HCl pure drug was accurately weighed and transferred to 10ml volumetric flask and one ml of 3%w/v of hydrogen peroxide was added and kept aside for two hrs and made up to volume with mobile phase. Then from this 10mcg/ml solution was prepared and injected in HPLC system to obtain chromatograms.
4. Photolysis
About 10mg of Tamsulosin HCl pure drug was accurately weighed and transferred to 10ml volumetric flask and made up to volume with mobile phase and kept aside for 8hr under direct sunlight. Then from this 10mcg/ml solution was prepared and injected in HPLC system to obtain chromatograms.
Results and Discusions
A reversed-phase chromatographic technique was developed to quantitate Tamsulosin HCl and it shows maximum absorbance (λmax) in acetonitrile at 275nm. In HPLC 275nmn is selected, peaks are seen at this wave lengths using T.D.W. and acetonitrile (60:40) as mobile phase with retention time 1.608. The flow rate was increased from 0.8ml/minute to 1ml/ minute. Merging of peaks was seen at flow rate 1ml/min. At flow rate 0.8ml/minute peaks were well resolved. It was our intention to use low flow rates as the high flow rates more than 1 decrease the life time of both the column and the pump. It is desirable to have retention times less than 10 minutes as this allows multiple analyses to be carried out in a reduced time. Celecoxib was taken as the internal standard and the retention time was found to be 2.767. In case of symmetrical peaks, peak heights are taken into consideration. In the present study we had choosen peak area ratio of drug/ internal standard. The present method was developed by taking peak area ratio of drug/ internal standard and validated. The marketed formulation is analyzed and the percentage recovery values of pure drug from the preanalyzed solution of formulation were in between 97.74% (Table 2). It was found that the drug obeys linearity within the concentration range of 1-200μg/ml and a straight line passing through the origin was observed (Figure 4). The LOQ was found to be 0.74μg/ml and the LOD was found to be 0.36μg/ml. The capacity factor was more than 2
The robustness of the assay method was established by introducing small changes in the HPLC conditions which included wavelength (273 and 277 nm), percentage of acetonitirile in the mobile phase (38 and 42%) and flow rate (0.7 and 0.9 mL min-1). Robustness of the method was studied using six replicates at a concentration level of 20 μg mL-1 of Tamsulosin HCL. The % RSD value of assay determined for the same sample under original conditions and robustness conditions was less than 2.0% indicating that the developed method was robust method was found to be simple, precise, accurate and robust for determination Tamsulosin HCl in pure and its dosage forms and also reveals that the commonly used excipients and additives in the pharmaceutical formulations were not interfering in the proposed method.
On Acid treatment (1ml of 0.1NHCl) it was found there was increase in peak resolution, shown by increase in AUC. The retention time was changed from 1.608 to 1.617 and the additional peaks observed are due to degradation products. The percent drug remaining after acid treatment was found to be 63.88 which was calculated from standard graph.
On Alkaline treatment (1ml of 0.1NNaOH) it was found there was increase in peak resolution, shown by decrease in AUC. The retention time was changed from 1.608 to 1.608 and the additional peaks observed are due to degradation products. The percent drug remaining after alkaline treatment was found to be 66.46 which was calculated from standard graph.
The 2 hr exposure to 3 % v/v H2O2 also affects the peak resolution much and the degradants are few and well separated from the drug peak.
When exposed to direct sunlight for 8 hrs, the retention time increased to 1.658 and the drug increases to 89.38 % of the initial amount. Although the peaks of degradants are well separated from drug peak, it’s better to keep the solutions in dark/in containers which cut off light.
Conclusion
The proposed method was found to be simple, precise, accurate and rapid for determination Tamsulosin HCl from pure and its dosage forms & validated as per ICH guidelines. The mobile phase is simple to prepare and economical. The sample recoveries in all formulations were in good agreement with their respective label claims and they suggested noninterference of formulation excipients in the estimation. Hence, this method can be easily and conveniently adopted for routine analysis of Tamsulosin HCl in pure form and its dosage forms and can also be used for dissolution or similar studies.
Acknowledgment
The authors are thankful to Dr. P Suresh, Dean and Principal, Gitam Institute of Pharmacy, GITAM university for providing necessary support.
References
- Martindale, The Extra Pharmacopoeia, Council of Royal Pharmaceutical Society of Great Britain, 29th edition, 1989.
- S.Budavari, The Merck Index, Merck and Co. Inc., White House Station, NJ, USA, 14th edition, 1996.
- Kakizaki H, Ameda K, Kobayashi S, Tanaka H and Shibata T Tomohiko Koyanagi, Urodynamic effects of α1-blocker tamsulosin on voiding dysfunction in patients with neurogenic bladder. Int. J. Urol. 2003; 10: 11576.
- Shrivastava A, Saxena P, Gupta VB, Spectrophotometric estimation of tamsulosin hydrochloride by acid-dye method. Pharmeceutical methods. 2011; 2(1): 53-59.
- Susmitha K, Radha K and Venkateshwarlu G. Extractive Spectrophotometric methods for determination of tamsulosin hydrochloride in pharmaceutical formulations using acidic tri phenylmethane dyes. Asian journal of Research in Chemistry. 2011; 4(7):1114-18.
- Nanda RK, Gaikwad J, Prakash A, Estimation of TamsulosinHCl and Tolterodine in its pharmaceutical dosage form by spectrophotometric method. Int. J. Pharm. Tech. Res. 2009; 1(3): 420-423.
- V.V. Pande, Simultaneous estimation of Tamsulosin hydrochloride and Dutasteride in combined dosage form by UV spectroscopy method. Journal of Pharmacy Research. 2009; 2(3): 507-509.
- Meiling Qi, Peng Wang, Rui-Hua Cong, Determination of the enantiomers of Tamsulosin hydrochloride and its synthetic intermediates by chiral liquid chromatography. Chromatographia. 2004; 59: 251-254.
- Patel DB, Patel NJ, Validated stability indicating HPTLC method for the determination of tamsulosin hydrochloride in pharmaceutical dosage forms. Int J Chem Tech Res. 2010; 2(1): 646-652.
- S. B. Bari, A. R. Bakhshi, P. S. Jain, and S. J. Surana, Development and Validation of Stability-Indicating HPTLC Determination of Tamsulosin in Bulk and Pharmaceutical Dosage Form. Chromatography Research International.2011;Volume 2011: 893260.
- J. Macek,J. Klíma, P. Ptác ek ,Rapid determination of tamsulosin in human plasma by high-performance liquid chromatography using extraction with butyl acetate. Journal of Chromatography B. 2004; 809 (2):307–311.
- G. Chandorkar, V. B. Kotwal, N. S. Dhande, S. G. Gurav, V. V. Pande, and P. V. Yadav, A sensitive HPLC method for simultaneous estimation of tamsulosin hydrochloride and its impurity. Pakistan Journal of Pharmaceutical Sciences. 2008; 21(3), 307–310.
- Richa Kumari, P. P. Dash, V. K. Lal, A. Mishra and P. N. Murthy, RP – HPLC method for the estimation of Tamsulosin Hydrochloride in Tablet Dosage Form. Indian Journal of Pharmaceutical Sciences. 2010; 2(6): 785–787.
- Sudha Thomas, Jitendra Dhomane, A validated RP-HPLC method for the determination of impurities in tamsulosin hydrochloride. Int JChem Res 2011; 2(4): 29-33.
- Fan HR, Gu Y, Si DY, Liu CX, Determination of tamsulosin in dog plasma by a high sensitive liquid chromatography tandem mass spectrometric method. Yao Xue Xue Bao. 2007; 42(8): 872-76.
- L. Ding, M. Li, P. Tao, J. Yang, and Z. Zhang, Quantitation of tamsulosin in human plasma by liquid chromatography-electrospray ionization mass spectrometry. Journal of Chromatography B. 2002; 767(1): 75–81.
- N. V. S. Ramakrishna, K. N. Vishwottam, S. Manoj, M. Koteshwara, S. Wishu, and D. P. Varma, Rapid, simple and highly sensitive LC-ESI-MS/MS method for the quantification of tamsulosin in human plasma. Biomedical Chromatography. 2005; 19(10): 709–719.
- Ksycinska H, Rudzki PJ, Sarosiek A, LC-MS Determination of Tamsulosin in human plasma and its application to pharmacokinetic study. Acta Pol. Pharm 2006; 63(5):417-419.
- Keski-Rahkonen P, Parssinen O, Leppanen E, Mauriala T, Lehtonen M, Auriola S, Determination of tamsulosin in human aqueous humor and serum by liquid chromatography-electro spray ionization tandem mass spectrometry. J Pharm Biomed Anal 2007; 43(2): 606-12.
- Matsushima H, Takanuki KI, Kamimura H, Watanabe Tand Higuchi S, Highly sensitive method for the determination of tamsulosin hydrochloride in human plasma dialysate, plasma and urine by high performance liquid chromatography- electro spray tandem mass spectrometry. Drug Metab. Dispos, 2004; 26(3): 240-245.
- Nageswra Rao R, Kumar Talluri MV, Narasa Raju A, Shinde DD, Ramanjaneyulu GS, Development of a validated RP-LC/ESI-MS-MS method for separation, identification and determination of related substances of tamsulosin in bulk drug and formulations. J Pharm Biomed Anal. 2008; 46(1): 94-103.
- ICH Q2A Guideline, Validation of Analytical Procedures: Definition and Terminology (CPMP III/ 5626/94), ICH, Geneva, Switzerland, 1995.
- ICH—Guidelines Q2B, Validation of Analytical Procedures: Methodology (CPMP/ICH/281/95), ICH, Geneva, Switzerland, 1996.
- ICH-Guidelines Q1A- Q1E, Stability Testing of New Drug Substances and Products, 2003.