Spectrofluorimetric assay method for glutathione and glutathione transferase using monobromobimane
- *Corresponding Author:
- S. I. Yakubu
Department of Clinical Pharmacy and Pharmacy Administration, University of Maiduguri, Nigeria.
E-mail: sanibnyakubu@googlemail.com
Date of Received: 28-12-2010
Date of Accepted: 06-06-2011
Available Online: 15-08-2011
Abstract
The primary role of glutathione transferase is to defend an organism from toxicities through catalyzing the reaction of glutathione (GSH) with potentially toxic compounds or metabolites to their chemically and biologically inert conju-gates. The objective of the study was to develop a simple and sensitive spectrofluorimetric assay method for glutathione transferase using monobromobimane (MBB), a non fluorescent compound with electrophilic site. MBB slowly reacted with glutathione to form fluorescent glutathione conjugate and that the reaction was catalysed by glutathione trans-ferase. Both non-enzymatic and enzymatic reaction products of MBB, in presence of GSH in phosphate buffer (pH 6.5), were measured by following increase of fluorescence at wavelength of 475nm. For validation of the assay method, the kinetic parameters such as the apparent Michaelis-Mente constants and maximum rates of conjugate formation as well as the specific activity of rat hepatic glutathione transferase were determined. The method was found to be sensitive, thus, applied to measure glutathione contents of crude preparation of rat hepatic cytosol fraction.
Keywords
glutathione, glutathione transferase, monobromobimane, spectrofluorimetric assay.
Introduction
Glutathione transferases are the most important family of enzymes involved in the metabolism of alkylating compounds and their metabolites. They are a major defence system in deactivating toxic materials (as parent compounds or metabolites) via glutathione conjugation within the body [1]. Studies [2,3] suggested that the action of glutathione transferases was to provide an activated glutathione for conjugation with any suitable electrophile, resulting in the product that is chemically and biologically inert. A study [4] indicated that glutathione and glutathione transferase are located mainly in the cytosol fraction of animal tissues and the enzyme activity is generally greater in hepatic than extra hepatic tissues. In the literatures, enzyme-catalysed conjugation of glutathione with an electrophile was followed by measurement of the amount of substrate reacted or product formed. Most of the methods utilized titrimetric [3,5] colorimetric [2] spectrophotometric [6,7,8], high performance liquid chromatography [9], or radioisotopic techniques [10]. Kosower et al [11] had previously reported that monobromobimane (a derivative of 1,5-diazobicyclo[3,3,0] actadiendiones) and non fluorescent compound with an electrophilic site, slowly reacted with glutathione to form fluorescent glutathione conjugate and that the reaction might be catalysed by glutathione transferase. Th us, the objective of the study was to exploit the fluorescent property of monobromobimane reaction product to develop a spectrofluorimetric assay method for glutathione transferase and to apply the method to determine glutathione contents of crude preparation of rat liver cytosol fraction.
Materials and Methods
Fluorescent instrumentation
Perkin-Elmer fluorescent spectrometer model 1000 set at excitation wavelength 475nm and its scale expansion gain control knob was adjusted so that a 10μM solution of the bimane conjugate gave a full scale reading of 100 fluorescence units. A Perkin-Elmer recorder model 56 attached to the spectrometer allowed recording of the change of fluorescence with time. Disposable plastic fluorescence cuvettes (Sarstedt, Number 67,754) were used throughout.
Preparation of standard stock solutions
a) The stock solution of glutathione (3 mM) was prepared by dissolving 90 mg of reduced glutathione (Sigma Aldrich, UK) in 100 ml of disodium ethylenediaminetetraacetic (EDTA) 10 Mm. The EDTA solution had been previously de-aerated with nitrogen gas.
b) The stock solution of monobromobimane (1.0mM) was prepared by dissolving 6.77 mg of monobromobimane in 25 ml of acetonitrile (HPLC grade).
c) Phosphate buffer solutions (0.05M) in the pH range 6.0-8.0 were prepared by adding varying amount of dilute sodium hydroxide (0.1 M) to separate aliquots (50ml) of potassium dihydrogen phosphate (0.1M) and diluted with distilled water to 100ml.
Preparation of liver cytoso1 fractions
Four Sprague-Dawley rats housed under the same conditions and fed on the same diet were sacrificed by cervical decapitation. Livers were excised quickly, trimmed, rinsed in ice-cooled 0.9% (w/v) saline to remove blood, blotted dry and weighed at temperature of 0 to 4°C. Each liver was homogenized in 3ml of 0.25 M sucrose per g of liver, and centrifuged at 3,000 and 10,000 rpm for 10 minutes respectively to precipitate cell debris. To the 10.000 rpm supernatant containing microsomes and cytosol fractions, 0.2 volume of 0.1 M calcium chloride solution was added, and further centrifuged at 10,000 rpm for 10 minutes to precipitate the microsomes. The resultant supernatant containing glutathione and its associate enzyme, glutathione transferase was diluted to a protein concentration of 810μg ml-1 [12].
Preliminary experiments
The purposes of the experiments were to determine the optimum pH of the buffer solution for non enzymatic and enzymatic reactions of monobromobimane (MBB) with glutathione (GSH) as well as the quenching effect of the presence of excess MBB in a typical reaction mixture on the fluorescence of the reaction product.
Determination of optimal pH
Non-enzymatic reactions were performed at 25°C. A typical reaction solution was prepared by addition of GSH (100 μl of the stock solution (3.0 mM) which gave a final concentration of 100 μM) to phosphate buffer (3 ml) in a fluorescent cuvette. The reaction was started by addition of MBB (30 μl of stock solution (1mM) which gave a final concentration of 10 μM). The development of fluorescence at 450 nm with time was followed on the chart recorder. The procedure was repeated with phosphate buffer of different pH values (6.0-8.0). A rate of increase of 1 fluorescence unit per minute on the recorder was calibrated to correspond to a rate of formation of conjugate of 0.1 nmoles ml-1 min-1. Blank cuvettes contained no GSH and showed an initial fluorescence reading of 1.5-2.2 units which did not significantly change with time.
Enzymatic reactions were performed using phosphate buffer of different pH values. The order of addition of reagents to the cuvette was as follows: buffer (3.0 ml), GSH (100 μl of the stock solution (3 mM) and enzyme (5 μl containing 360 μg proteins-1 ml-1). Addition of MBB (30 μl of stock solution (1mM)) started the reaction and was followed as above. True enzymatic rates were obtained by subtracting the non-enzymatic rates (no enzyme present) from the total rates given by these experiments.
Quenching experiment
The blank cuvette contained 3 ml of phosphate buffer (pH 6.5) to which aliquots (30 μl) of stock solution of MBB (1mM) were repeatedly added until a total of 900 μl of MBB was added. After each addition the fluorescence of the cuvette was measured. The above experiment was repeated in presence of 10 μl of liver supernatant (360 μg protein ml-1) that contained glutathione transferase and small amount of GSH. This GSH (final concentration in cuvette of 2.8 μM) reacted enzymatically with MBB (30μl; 10 μM concentration) to give the fluorescent conjugate (28 fluorescence units). Further repetitive additions of MBB were carried out as above. These additions increased the concentration of MBB surrounding the fluorescent conjugate. The differences between the fluorescence recorded for the blank experiment and the experiment containing conjugate at each concentration of MBB gave the fluorescence of the conjugate at that concentration of MBB.
Determination of Michaelis-Menten constant for monobromobimane
For validation of the assay, the enzymatic reaction between monobromobimane and glutathione was characterised to determine kinetic parameters such as apparent Michaelis-Menten constant and maximum rate of the reaction. Control experiments were included as the non-enzymatic rates of reaction.
In these experiments the concentration of glutathione (GSH) was kept constant at 100μM, and concentration of monobromobimane (MBB) was varied. The pH of the solutions was 6.5 and the temperature was 25°C.
In the reaction mixtures without enzyme, varying amounts of MBB (0-300 μl of 1 mM stock solution which gave final concentration 0-100 μM) were added into different fluorescent cuvettes already containing 3.0 ml of phosphate buffer (pH 6.5) and 100 μl of GSH stock solution (3 mM). Each cuvette was inverted three times to ensure complete mixing and the initial rate of product formed was followed by spectrofluorimetric measurement of increase in fluorescence per minute at wavelength 475nm.The rate represented the non enzymatic rate of product formed.
The procedure was repeated with the reaction mixture containing solution of enzyme (glutathione transferase (GTs)). Varying amounts of monobromobimane stock solution (0-300 μl) were added to respective cuvettes already containing 5 μl of the enzyme solution (protein concentration of 810 μg ml-1), glutathione (100 μl of stock solution) and phosphate buffer (3.0 ml). From the above experiments, total enzymatic rates (actual enzymatic plus non-enzymatic rates) were obtained.
Determination of Michaelis-Menten constant for glutathione
In these experiments, the concentration of monobromobimane was kept constant at 50 μM, and concentration of glutathione was varied. The pH of the solutions was 6.5 and the temperature was 25°C.
In reaction mixture without enzyme solution, varying amounts of glutathione (0-300 μl of stock solution giving 0-260 μM final glutathione concentrations) were reacted with 150 μl of monobromobimane solution (final concentration 50 μM) in presence of phosphate buffer (3.0 ml) in cuvettes. Initial rates of product formed were respectively followed by spectrofluorimetric measurement of increase in fluorescent per minute at 475nm.
The procedure was repeated for total enzymatic rates of product formed using reaction mixtures containing, in addition, aliquots (5 μl) of glutathione transferase solution (protein concentration 810 μg/ml). The results were as shown in Figures 5 to 8. The apparent Km value for glutathione and Vmax were estimated.
Determination of the glutathione contents of crude preparation of rat liver cytosol.
Glutathione is tripeptide that is present in nearly all living cells mainly in the cytosol with the highest concentration in the liver compared to other tissues of the body [4].
Treatment of animals
Four groups of Sprague-Dawley rats housed under the same conditions and fed on the same diet were used in this experiment. Animals in the first group were not treated with any drug prior to sacrifice. Animals in the second group were treated with one intraperitoneal injection of polychlorinated biphenyl (Aroclor 1254) 500 mg kg-1 and were sacrificed after 7 days, while those in the third group were treated with intraperitoneal injection of 3-methylcholanthrene 20 mg-1 kg-1day-1 for two days and were sacrificed 48 hours after treatment. Th ose animals in the fourth group were treated with 0.1% phenobarbitone in drinking water for 5 days and were sacrificed 24 hours after the last treatment. All animals were sacrificed by cervical decapitation and livers collected. Liver cytosol fraction from each group of rats was prepared according to Lowry et al [12] method described above.
Procedure:
Initially an experiment was performed that demonstrated the presence of glutathione in the liver cytosol fraction. In order to determine the GSH content, four preparations of liver cytosol fractions (10 μl) from different group of rats were respectively added into various cuvettes containing monobromobimane (30 μl stock solution; 10 μM concentration) in presence of phosphate buffer (3.0 ml) at pH 6.5 at 25°C. In the blank cuvette, distilled water (10 μl) was added to monobromobimane (stock solution 30 μl) in presence of phosphate buffer (3.0 ml). Each cuvette was inverted three times to ensure complete mixing and was allowed 10 minutes for reaction to proceed to completion. Increase of fluorescence was measured using spectrofluorimeter set at wavelength 475 nm. Subsequently, varying amounts of GSH (stock solution 3mM; final concentrations 1-10 μM) were added into the respective cuvettes containing the reaction mixtures and the increases of fluorescence were measured. The increase of fluorescence due to GSH conjugate was converted to the corresponding concentration of GSH in the reaction mixture since 1.0 fluorescence unit recorded on the chart had been calibrated to correspond to 0.1 μM concentration of GSH conjugate.
Results
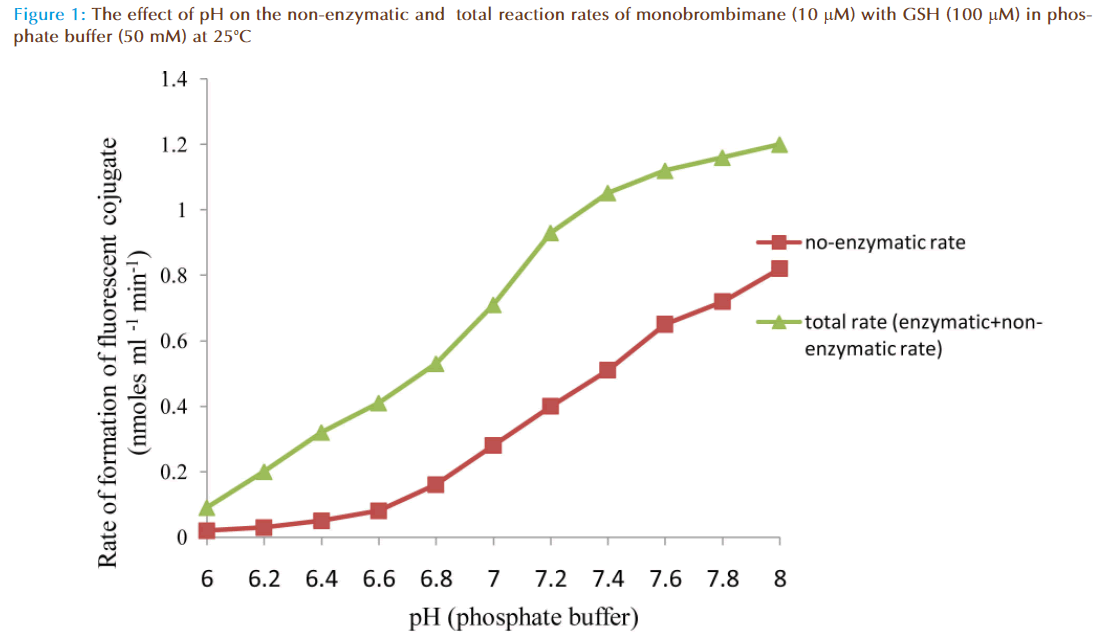
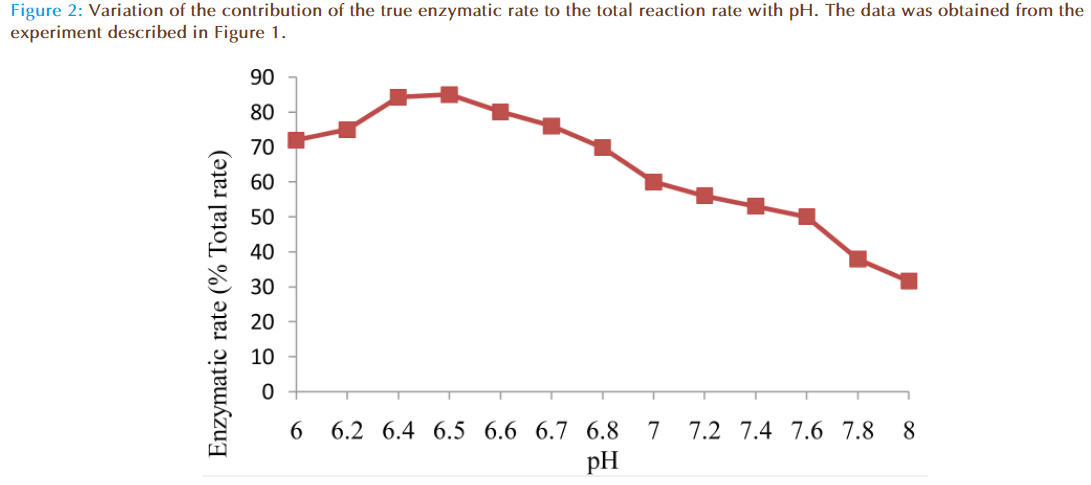
The results of the preliminary experiments showed increase of rate of reaction with increasing value of pH for non-enzymatic and enzymatic reactions. Also addition of solution of glutathione (GSH) transferase in the reaction mixture caused a marked increase in the rate of reaction compared to the non-enzymatic, showing monobromobimane was a substrate for this enzyme (Figure 1). A plot of enzymatic rate as percentage of the total reaction rate against pH showed the contribution by the true enzyme. The optimum was achieved at pH 6.5 (Figure 2).
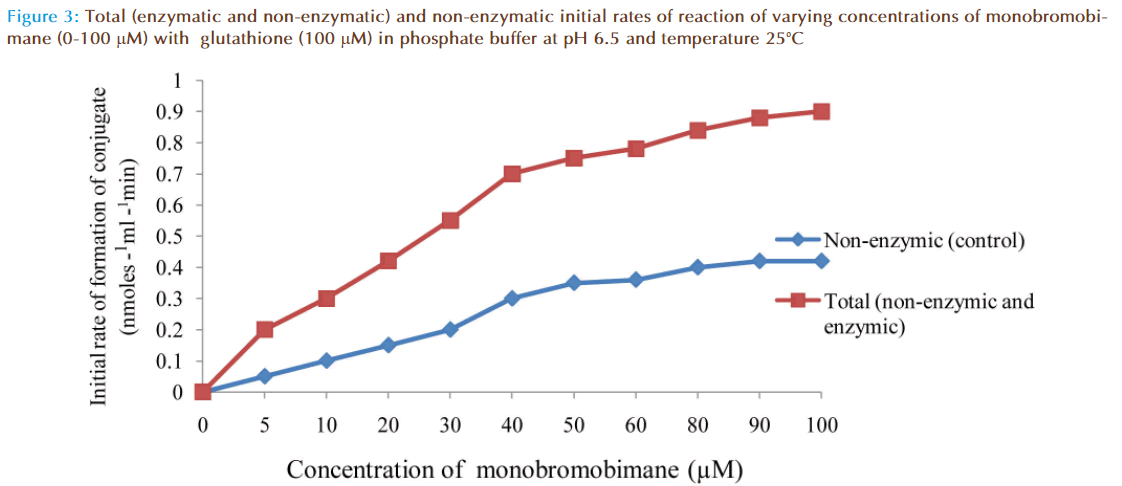
The effect of increasing monobromobimane (MBB) concentration on the rate of reaction between GSH and MBB was investigated and the results were as shown in Figure 3. The non-enzymatic rate increased linearly as expected with increasing MBB concentration due to increased formation of fluorescent conjugate until the concentration of MBB reached 50 μM. The non-enzymatic rate then apparently decreased. This decrease from the expected fluorescent conjugate might be due to quenching of the fluorescence by excessive amount of MBB (greater than 50 μM) surrounding the conjugate. The total rate of reaction was that obtained in the presence of a small amount of the enzyme. The total rate included true enzymatic and non-enzymatic rates. Addition of a large quantity of the enzyme would have increased the enzymatic contribution of the total rate of reaction mixture but that would have made the rate too fast to measure accurately and conveniently, especially at high MBB concentration. At the lowest monobromobimane concentration, care was taken to ensure that rates were measured at the initial portions of the progress curves on the chart-recorder.
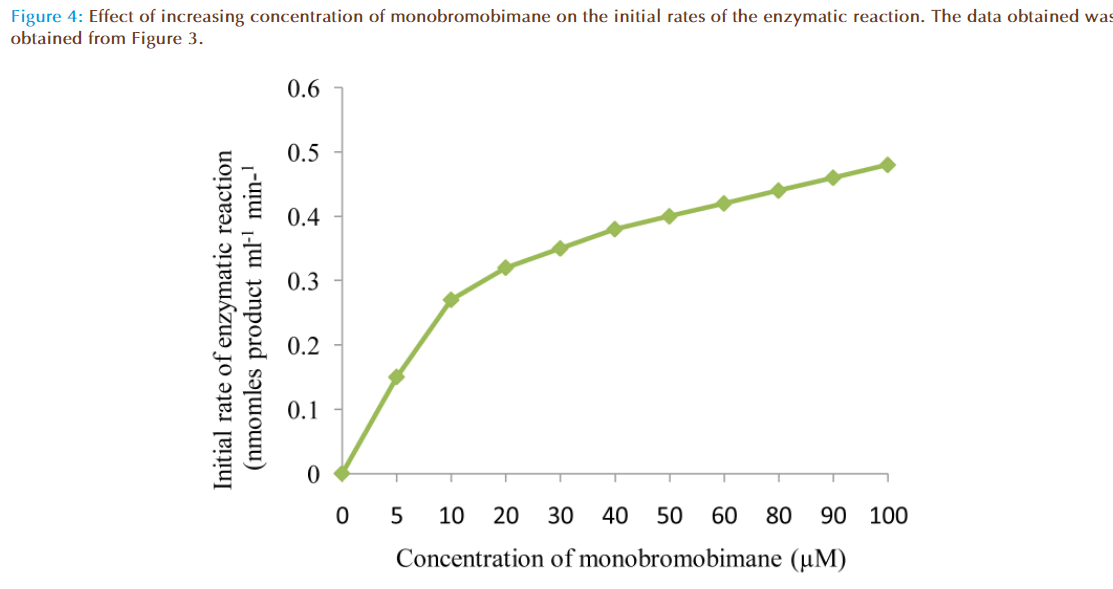
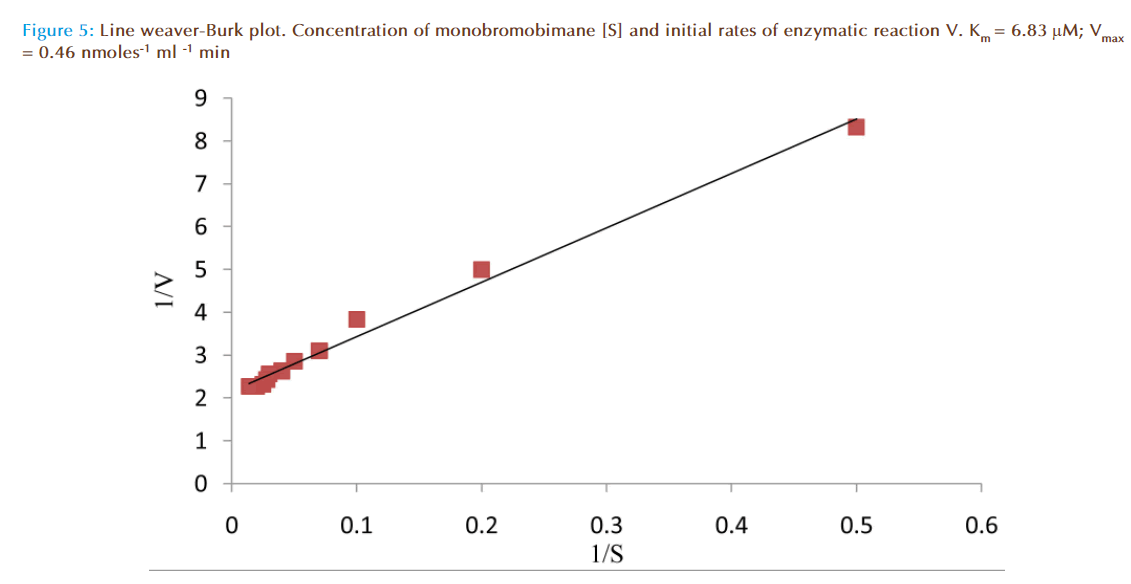
Figure 4 represented the true enzymatic rate obtained by subtracting the nonenzymatic rate from the total rate (non-enzymatic and enzymatic) at different concentrations of MBB. The data were obtained from Figure 3. The shape of the curve was as expected for an enzyme-catalysed reaction showing that saturation of the enzyme reaction occurred at higher concentrations of MBB. This demonstrated that MBB is a substrate. The data from Figure 4 were re-plotted to obtain linear plots. Figure 5 is a Lineweaver-Burk plot. The apparent Michaelis-Mentes constant (Km), maximum velocity for the enzyme preparation (Vmax) and specific activity of the enzyme fraction obtained from the plot were 6.83 μM, 0.46 nmoles ml reaction mixture-1 min-1 and 0.34 nmoles μg protein-1 min-1. The specific activity was calculated from the final protein concentration (1.3 nM μg protein-1 min-1) in the reaction mixture. While Figure 6 is Hofstee linear plot, the Km and (Vmax) obtained from the plot were 7.58 μM and 0.47 nmoles ml-1min-1. The kinetic parameters were in agreement for both plots.
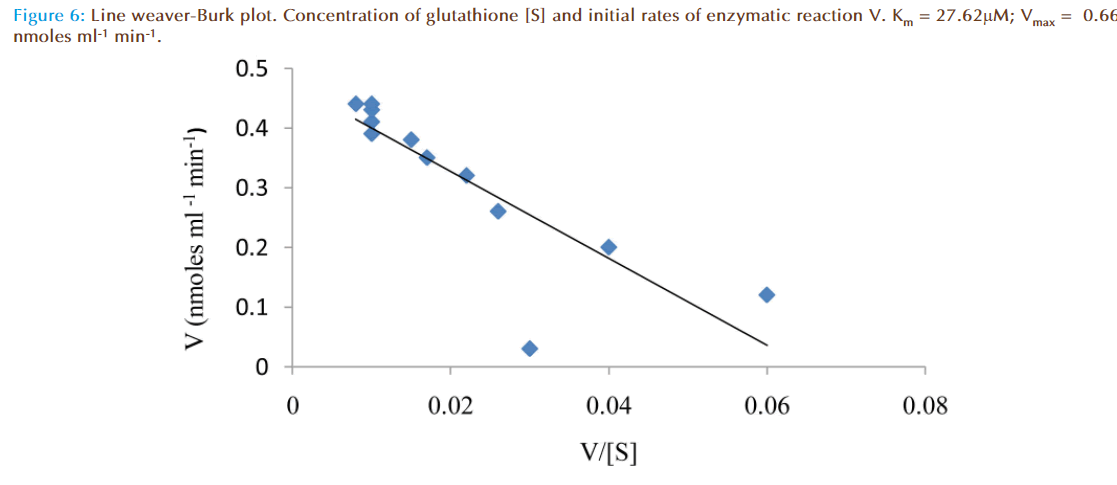
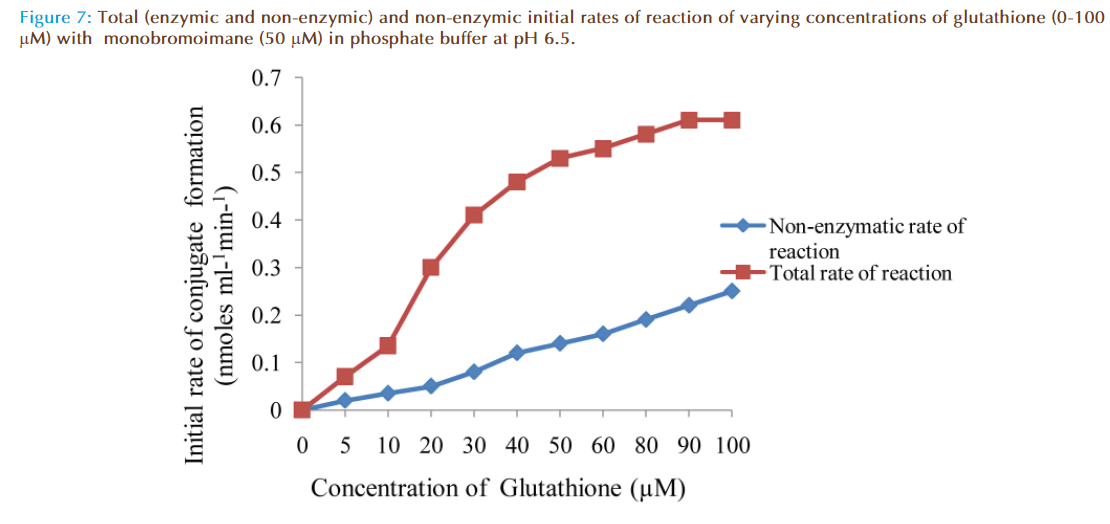
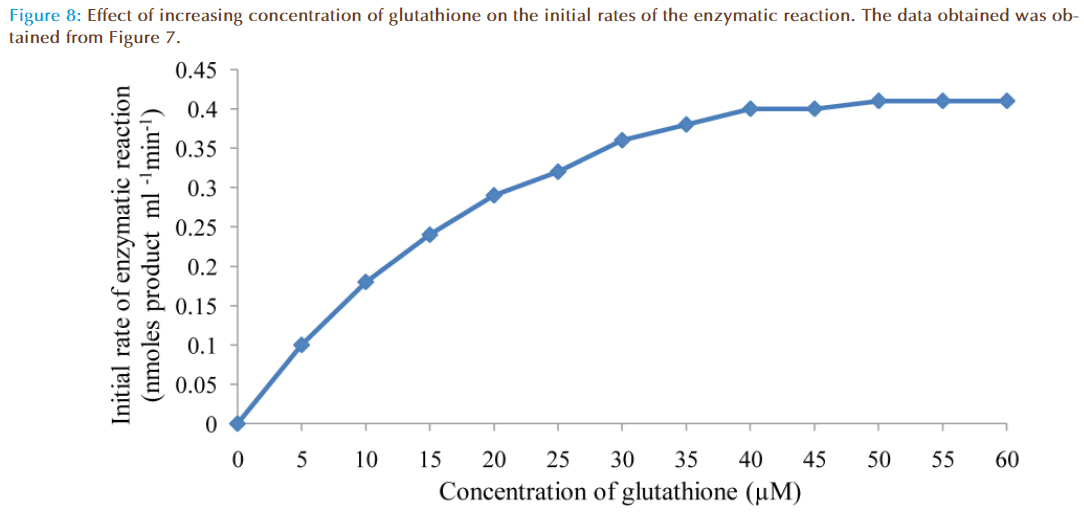
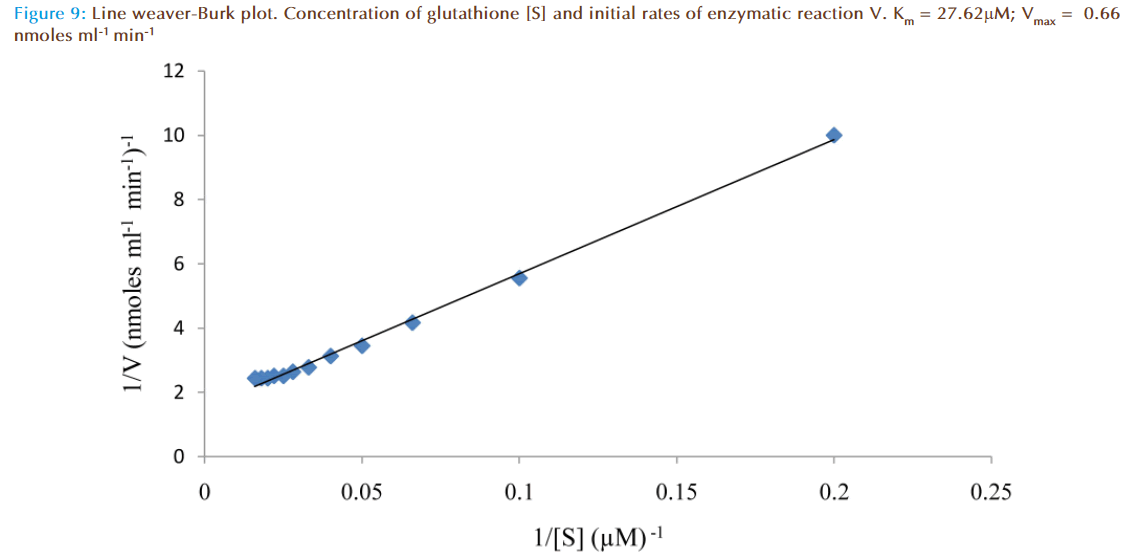
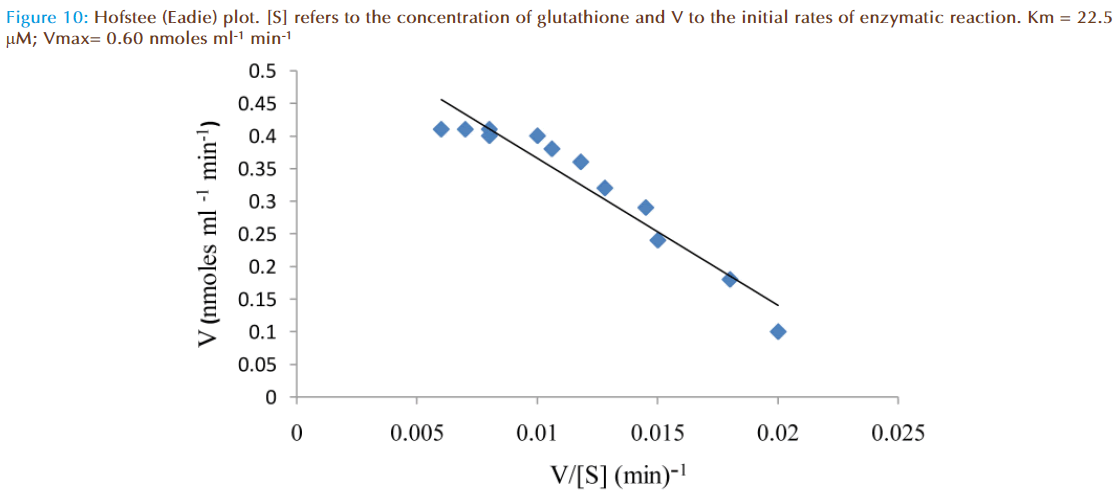
The effect of increasing glutathione concentration (at constant concentration of monobromobimane 50 μM) on the rates of reaction was similarly investigated and the results were as shown in Figure 7. Figure 8 represented the true enzymatic rate obtained by subtracting the non-enzymatic rate from the total rate in Figure 7 at different concentrations of glutathione. The results from figure 8 were re-plotted in Figures 9 and 10 to obtain Lineweaver-Burk and Hofstee linear plots respectively. Approximation of the slopes and intercept allowed estimation of apparent Km and Vmax values from Lineweaver-Burk and Hofstee linear plots were: 27.62 μM and 0.66 nmoles ml-1 min-1; 22.5 μM and 0.66 nmoles ml-1 min-1. These values for glutathione from the linear plots were in agreement.
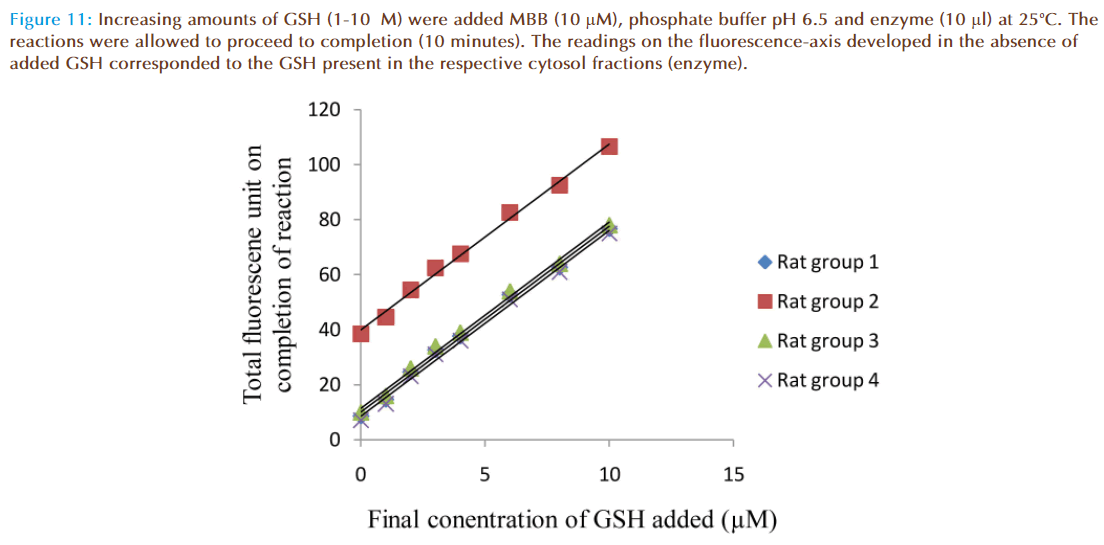
When solutions of MBB (10 μM), enzyme supernatant (10 μl) and varying concentrations of GSH (0-10 μM) were mixed, increase of fluorescence was observed. Fluorescence intensity increased with increasing concentrations of GSH (Figure 11). The linear graphs intercepted on the fluorescence –axis at 8.6, 38.5, 10 and 7.0 units respectively. These values corresponded to fluorescence units due to conjugate produced when enzyme preparation alone was mixed with MBB in each of the cuvettes, indicating GSH was present in the enzyme preparation (cytosol fraction). For determination of glutathione content of rat liver cytosol, the increase of fluorescence due to GSH conjugate was converted to corresponding concentration of glutathione in the reaction mixture since 1.0 fluorescence unit recorded on the chart corresponded to 0.1 μM concentration of GSH conjugate.
Figure 11: Increasing amounts of GSH (1-10μM) were added MBB (10 μM), phosphate buffer pH 6.5 and enzyme (10 μl) at 25°C. The reactions were allowed to proceed to completion (10 minutes). The readings on the fluorescence-axis developed in the absence of added GSH corresponded to the GSH present in the respective cytosol fractions (enzyme).
Wet weight (mg) of liver per ml of total homogenate for each group of rats was calculated and using this value and concentration of GSH in liver cytosol fraction for each group, the GSH content of liver (μmoles g-1 wet weight) was determined as presented in Table 1.
Discussion
Titrimetric, colorimetric, spectrophotometric or radioisotopic techniques of analysis have been used in the study of glutathione (GSH) and glutathione transferase. In this study an alternative method of analysis using spectrofluorimetric techniques has been examined. Monobromobimane (MBB), relatively non-fluorescent compound with an electrophilic centre, is a derivative of strongly fluorescent compound bimane (1, 5-diazabicyclo 3.3.0 octadienediones). Chemically, MBB reacts with the sulphydryl (-SH) group of glutathione to form fluorescent product. Addition of a small amount of glutathione transferase (GTs) to the reaction mixture of MBB and GSH resulted in a marked increase of fluorescence compared to the mixture without enzyme, demonstrating MBB is a substrate for the enzyme.
Preliminary investigation has revealed quenching of fluorescence of reaction product by MBB at high concentrations. Initially, the fluorescence of reaction product increased proportionally with increase of concentration of MBB in accordance with Beer-Lambert law. However, at high concentrations of MBB beyond (100 μM), the fluorescence started to decrease with increasing concentrations. Therefore, for valid quantitative analysis, concentrations of MBB (1-100 μM) were used.
The effects of pH on rates of non-enzymatic and enzymatic conjugation of glutathione with MBB were studied. Glutathione ionized in aqueous buffer solution to GS- and H+. This ionization increased with an increase of pH value. Both rates of non-enzymatic and enzymatic reaction of GSH with MBB were observed to increase with the increasing value of pH (6.0-8.0) of the buffer solution. A plot of actual enzymatic rate (expressed as percent of total enzymatic rate) against pH values indicated the rate was maximal at pH 6.5 (Figure 2). At pH value less than 6.5, the decrease in the enzymatic rate of product formation might be attributable to acid de-naturation of protein thereby reducing its catalytic capability. While at pH value greater than 6.5, the decrease might be due increased ionization of GSH which made the non-enzymatic rate product formation much rapid to upset the enzymatic rate. Th us, the pH optimum for the conjugation of GSH with MBB by glutathione transferase was 6.5.
GS- and H+. This ionization increased with an increase of pH value. Both rates of non-enzymatic and enzymatic reaction of GSH with MBB were observed to increase with the increasing value of pH (6.0-8.0) of the buffer solution. A plot of actual enzymatic rate (expressed as percent of total enzymatic rate) against pH values indicated the rate was maximal at pH 6.5 (Figure 2). At pH value less than 6.5, the decrease in the enzymatic rate of product formation might be attributable to acid de-naturation of protein thereby reducing its catalytic capability. While at pH value greater than 6.5, the decrease might be due increased ionization of GSH which made the non-enzymatic rate product formation much rapid to upset the enzymatic rate. Th us, the pH optimum for the conjugation of GSH with MBB by glutathione transferase was 6.5.
At a constant concentration of GSH with varying concentrations of MBB, the apparent Km, Vm and specific activity were respectively determined to be (8 to 9) μmolar; (0.44 to 0.48) nmoles ml reaction mixture-1 min-1; and 0.37 nmoles μg protein-1 min-1. However, at a constant concentration of GSH with varying concentrations of GSH, Lineweaver-Burk and Hofstee plots of initial velocity as function of glutathione concentration revealed apparent Km and Vm values: 27.62 μM and 0.66 nmoles ml-1 min-1 respectively.
Following the validation, the spectrofluorimetric assay method was utilized to determine glutathione content of rat livers. The protection of tissues by glutathione from the toxic effects of alkylating agents and pharmacologically active compounds has long been reported by several workers. Depletion of GSH levels necessary for adequate protection to less than 20-30% has been suggested as an explanation of hepatotoxicity of higher doses of compounds such as paracetamol or bromobenzene [13]. Kwizer et al [7] have examined the effects of hepatotoxic drugs such as paracetamol, cocaine, dextropropoxyphene and hycanthone on glutathione content of isolated rat hepatocytes and reported that addition of a hepatotoxic drug caused a rapid fall in glutathione content. The glutathione content of the hepatocytes was determined by Ellman`s method using spectrophotometer. Chung et al [8] has reported the use of a modified spectrophotometric assay for GSH, based on an enzymatic recycling reaction, using Ellman`s reagent to determine GSH status in rat liver. In another study, Vogt and Richie [14] have examined the effect of ethanol administration in the aging mouse on GSH content. The GSH content was assayed enzymatically using high performance chromatography (HPLC).
In this study, the values of GSH content of liver were determined by spectrofluorimetric assay method (Table 1). The values were within acceptable range (6-10) μmoles per gramme wet weight of liver consistent with those reported by Jakoby et al [6]. The differences between the individual values of GSH content of liver determined in this assay were small, thus, confirming the literature report that GSH content of liver did not change when an animal was pretreated with microsomal drug-metabolizing inducers such as phenobarbitone, 3-methylcholanthrene and polychlorinated biphenyl (Arcolor 1254). Therefore, the new spectrofluorimetric assay method for GSH and its associated enzyme using monobromobimane can be an alternative in biochemical studies of drug metabolism and drug toxicity.
| Wet weight (mg) ofliver/ml of the totalhomogenate | Increase influorescence due toliver cytosol fraction(10μl) | Concentration of GSH in reactionmixture (μM) | Concentration of GSH in cytosol fraction(mM) | GSH content of liver μmoles g-1wet weight |
|---|---|---|---|---|
| 51.38 | 8.60 | 0.86 | 0.27 | 5.3 |
| 160.00 | 38.5 | 3.85 | 1.21 | 7.6 |
| 48.90 | 10.00 | 1.00 | 0.31 | 6.4 |
| 61.70 | 7.00 | 0.70 | 0.22 | 5.6 |
Table 1: GSH content of rat liver determined by spectrofluorimetric assay method
Conclusion
The new spectrofluorimetric assay method for GSH and glutathione transferase activity using monobromobimane (MBB) could among other methods be used as a sensitive and specific research tool for biochemical studies of drug metabolism and drug toxicity.
References
- Boyland E and Nery R. Mecapturic acid formation during the metabolism of arecoline and arecaidine in the rat. Biochem. J., 113, 123-130, (1969).
- Al-Kassab S, Boyland E, and Williams K. An enzyme from rat liver catalysing conjugations with glutathione. 2. Replacement of nitro groups. Biochem. J.1963;87:4-9.
- Eaton DL, Bammler TK. Glutathione S-transferases. In: Levy RH, Thummel KE, Trager WF et al. eds. Metabolic Drug Interactions. Philadelphia, PA: Lippincott,Williams and Wilkins; 2000.
- Marchand DH, Remmel RP, Abdel-Monem MM. Biliary excretion of a glutathione conjugates of busulfan and 1, 4-diiodobutane in the rat. Drug Metab Dispos. 1988; 16(1): 85–92.
- Booth, J. Et al. An enzyme from rat liver catalysing conjugations with glutathione. Biochem. J. 1961; 79: 516-524.
- Jakoby, W.B. In: Glutathione metabolism and function. Raven Press, New York. 1976; pp. 189-202.
- Kwizera EN, Hulbert PB and Hicks R. Depletion of the glutathione content of isolated liver cells by hepatotoxic drug. Paper presented at British Pharmaceutical Conference, Newcastle upon Tyne September, 1980.
- Chung FL Komninou D, Zhang L, Nath R, Pan J, Amin, S, Richie, J. Glutathione depletion enhances the formation of endogenous cyclic DNA adducts derived from t-4-hydroxy-2-nonenal in rat liver, Chemical Research in Toxicology. 2005; vol. 18, No. 1, pp. 24-27.
- Kleinman WA, Richie J JP. Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol 2000; 60:19–29.
- Yukiko Kimura, Measurement of glutathione synthesis by isotope ratio mass spectrometry in systemic inflammation, PhD Thesis 2010, Department of Paediatric Surgery, Institute of Child Health University College London.
- Kosower EM, Pazhenchevsky B and Hershkowitz E. J. Amer. Chem. Soc 1978; 100: 6516-6518.
- Lowry OH, Rosebrough NJ, Farr AL and Randall, R.J., J. Biol. Chem. 1951; 193: 265-275.
- Mitchell JR, Jallow DJ, Potter WZ, Gillette JR and Brodie BB. Acetaminophen- induced hepatic necrosis. IV: proactive role of glutathione. J. Pharmacol. Exp. Therap. 1973; 187, 211-217.
- Vogt, BL and Richie, JP. Glutathione depletion and recovery after acute ethanol administration in the aging mouse. Biochem Pharmcol 2007; 70: 1613- 1621.