Screening of selected biological activities of Artocarpus lakoocha Roxb (Moraceae) fruit pericarp
- *Corresponding Author:
- H. L. Raghavendra
Department of Biochemistry, P.G Centre, Kuvempu University, Shivagangothri, Davangere, Karnataka, India
E-mail: raghavendra.hl@rediffmail.com
Date of Received: 26-06-2010
Date of Modified: 30-07-2010
Date of Accepted: 04-10-2010
Available Online: 15-11-2010
Abstract
Artocarpus lakoocha Roxb (Moraceae) is cultivated in Uttar Pradesh, Bengal, Khasi Hills and Western Ghats. Objectives of the present study were to de-termine antibacterial, antioxidant, anthelmintic and insecticidal efficacy of methanol extract of A. lakoocha fruit pericarp. Antibacterial activity was tested against by Agar well diffusion method. Antioxidant activity in terms of free radical scavenging ability was determined by DPPH free radical scavenging assay. Anthelmintic efficacy was determined using adult Indian earthworm. Insecticidal activity was tested against sec-ond and third instar larvae of Aedes aegypti. The extract has shown dose depend-ent antibacterial, antioxidant, anthelmintic and insecticidal activity. Among bacteria, S. aureus has shown more susceptibility than K. pneumoniae and P. aeruginosa. The extract exhibited marked antioxidant activity by scavenging DPPH free radical. The IC50 value for extract was found to be 49.42μg/ml. The extract exhibited marked an-thelmintic activity by causing paralysis and death of worms and the effect was found to be dose dependent. The extract concentration 100mg/ml has shown marked an-thelmintic effect than standard drug. In insecticidal study, the 2nd instar larvae were shown to be more susceptible than 3rd instar larvae. Phytochemical analysis revealed the presence of tannins and alkaloids. The presence of these phytoconstituents might be responsible for the biological activities of extract tested. The extract could be used to treat free radical damage, bacterial and helmintic infections and to control insect vectors. Further studies on isolation of constituents and their bio-efficacies in vitro and in vivo are under investigation.
Keywords
Artocarpus lakoocha Roxb, Agar well diffusion, Pheretima pasthuma, DPPH, IC50, Aedes aegypti
Introduction
Artocarpus lakoocha Roxb (Syn: A. lacucha Buch.-Ham.) is a member of the family Moraceae and is cultivated in Uttar Pradesh, Bengal, Khasi Hills and Western Ghats. It is called Monkey Jack in English and in Ayurveda it is called Lakuch, Kshudra Panas, Granthiphala and Pitanaasha. Bark when applied externally, draws out purulent matter; heals boils, cracked skin and pimples. Seeds are purgative, haemagglutinating. Stem is vermifuge. The stem bark contains oxyresveratrol, used for tapeworm. A lectin, artocarpin, isolated from seeds, precipitates several galactomannans. It agglutinates rat lymphocytes and mouse ascites cells [1]. The lakoocha fruits are generally eaten fresh. The edible fruit pulp is believed to acts as a tonic for the liver. The raw fruits and male flowers spikes (acidic and astringent) are utilized in pickles and chutney. The brown powder called Puag-Haad in Th ailand is a product of the aqueous extraction of A. lakoocha prepared by boiling the wood chips and then evaporating water away. Th is preparation has been used as a traditional anthelmintic drug for treatment of tapeworm infection in Th ailand [2,3]. The hardwood sold as lakuch is comparable to famous teak wood, is used for constructions, furniture, boat making and cabinet work. Tree bark containing 8.5% tannin is chewed like betel nuts and is also used to treat skin ailments. It yields a durable fiber good for cordage. The wood and roots yield a lavish color dye [4]. Two isolectins, ALA-I and ALA-II, isolated from seed extracts of A. lakoocha possessed several similar properties such as blood type agglutination, pH optimum, pH and temperature stability, as well as binding specificity towards asialomucins [5]. Two new stilbene derivatives, lakoochins A and B, were isolated from the roots of A. lakoocha. Both exhibited antimycobacterial activity and showed cytotoxic activity against some cell lines [6]. Oxyresveratrol, isolated from heartwood of A. lakoocha has shown moderate anti-herpes simplex virus activity and anti-HIV activity against a wild-type human immunodeficiency virus type 1 [7]. Critical review of literature revealed scanty information on antibacterial, antioxidant, anthelmintic and insecticidal activity of fruit pericarp of A. lakoocha. Th us, the present investigation has been carried to investigate antibacterial, antioxidant, anthelmintic and insecticidal activity of fruit pericarp of A. lakoocha.
Experimental
Collection and identification of plant material
The fruits of A. lakoocha were collected during April 2010 from outskirts of Shivamogga, Karnataka, India. The fruit was identified by specialist and a voucher specimen (SRNMN/PK/Al-801) was deposited in the department for future reference.
Drugs and Chemicals used
Methanol (HiMedia, Mumbai), Dimethyl sufloxide (S.D Fine Chemicals, Mumbai), Rifampicin (Ranbaxy Laboratories, New Dehli), Piperazine citrate (GlaxoSmithKline Pharmaceutical Limited, Bangalore), 2,2-Diphenyl-1-Picrylhydrazyl (DPPH, Sigma Chem. Co., USA).
Extraction and Phytochemical analysis
The collected ripe fruits were washed thoroughly, pericarp was separated, shade dried and powdered using blender. For extraction, the powdered material was subjected to soxhlet extraction and exhaustively extracted with methanol for about 48 hours. The extract was filtered, concentrated using rotary flash evaporator and dried in the desiccator. The extract was subjected to preliminary phytochemical screening to detect secondary metabolites [8,9].
Preparation of extract for antibacterial, anthelmintic and insecticidal activity
The concentrated methanol extract was dissolved in 10% Dimethyl sulfoxide (DMSO) to get concentrations 10, 25, 50 and 100mg/ml.
Screening for Antibacterial activity of methanol extract
The antibacterial efficacy of methanol extract of pericarp was tested against Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa by Agar well diff usion method [10]. Briefly, 24 hours old broth cultures of test bacteria were swabbed on sterile Muller- Hinton agar plates using sterile cotton swab followed by punching wells of 6mm with the help of sterile cork borer. The Standard drug (Rifampicin, 1mg/ml of sterile distilled water) and Control (10%DMSO) and diff erent concentrations of extract were added to respectively labeled wells. The plates were incubated at 37°C for 24 hours in upright position and the zone of inhibition was recorded. Experiment was carried thrice and average reading was noted.
Screening for free radical scavenging ability of methanol extract
The radical scavenging ability of methanol extract and the Ascorbic acid (standard) was tested on the basis of the radical scavenging eff ect on the DPPH free radical [11]. Diff erent concentrations of extract and standard namely 25, 50, 100, 200 and 400μg/ml were prepared in methanol. In clean and labeled test tubes, 2 ml of DPPH solution (0.002% in methanol) was mixed with 2 ml of diff erent concentrations of extract and standard separately. The tubes were incubated at room temperature in dark for 30 minutes and the optical density was measured at 517nm using UV-Vis Spectrophotometer. The absorbance of the DPPH control was also noted. The scavenging activity of the extract was calculated using the formula
Scavenging activity (%) = A – B / A × 100
where A is absorbance of DPPH and B is absorbance of DPPH and extract/standard combination. The IC50 value was calculated which denotes the concentration of extract required to scavenge 50% of DPPH free radicals.
Screening for Anthelmintic activity of methanol extract
In this study, adult Indian earthworm Pheretima pasthuma was used to assess anthelmintic potential of methanol extract of pericarp due to its anatomical and physiological resemblance with the intestinal roundworm parasite of human beings. The worms were identified in the department of Zoology, Sahyadri Science College (Autonomous), Shivamogga. Standard drug (Piperazine Citrate, 1%) and diff erent concentrations of methanol extract (10, 25, 50 and 100mg/ml of 10% DMSO) were poured into respective labeled petriplates containing saline. A saline control was kept. Six worms of nearly equal size were introduced into each of the plates. Observations were made for the time taken to paralysis and death of individual worm. Paralysis was said to occur when the worms were not able to move even in normal saline. Death was concluded when the worms lost their motility followed with fading away of their body colors. Death was also confirmed by dipping the worms in slightly warm water. The mortality of parasite was assumed to have occurred when all signs of movement had ceased [12,13].
Screening for Insecticidal activity of methanol extract
The insecticidal efficacy of methanol extract of pericarp was determined against second and third instar larvae of Aedes aegypti. The larvae were colcollected from stagnant water and identified in University of Agricultural Sciences, Shivamogga by an Entomologist. Briefl y, diff erent concentrations of methanol extract (10, 25, 50 and 100mg/ml of 10% DMSO) were added to labeled beakers containing twenty larvae. A beaker containing only water (i.e., without extract) serves as control. The larvicidal effect of the extract was determined by counting the number of dead larvae after 24 hours and the observation was continued for up to 72 hours. Dead larvae were identified when they failed to move after probing with a needle in siphon or cervical region. The test was repeated thrice and the percentage of larval mortality for each concentration of extract was calculated [14].
Results
The preliminary phytochemical analysis of methanol extract of A. lakoocha fruit pericarp revealed the presence of tannins and alkaloids.
The result of antibacterial activity of methanol extract of fruit pericarp is shown in Table 1. Results were recorded as presence or absence of zones of inhibition around the well. The inhibitory zone around the well indicated the absence of bacterial growth and it as reported as positive and absence of zone as negative. In this study, the extract has shown inhibition of test bacteria in a concentration dependent manner. Among bacteria, S. aureus was found to be more susceptible to extract followed by K. pneumoniae and P. aeruginosa. Standard antibiotic caused more inhibition of test bacteria than methanol extract. No inhibition of test bacteria was observed in case of control i.e., 10% DMSO. It appears that overall the bacteria were found to be sensitive to extract. The reasons for this could be that the components from the plant active against microorganisms are most often obtained through solvent extraction.
| Treatment | Concentration | Inhibition Zone in cm | ||
|---|---|---|---|---|
| S. aureus | K. pneumoniae | P. aeruginosa | ||
| Control | 10% | 0.0 | 0.0 | 0.0 |
| Standard | 1 mg/ml | 3.8 | 3.8 | 3.9 |
| 10 mg/ml | 1.1 | 0.9 | 0.8 | |
| 25 mg/ml | 1.5 | 1.3 | 1.3 | |
| Methanol extract | ||||
| 50 mg/ml | 1.9 | 1.7 | 1.6 | |
| 100 mg/ml | 2.2 | 1.9 | 1.8 | |
Table 1: Antibacterial activity of methanol extract
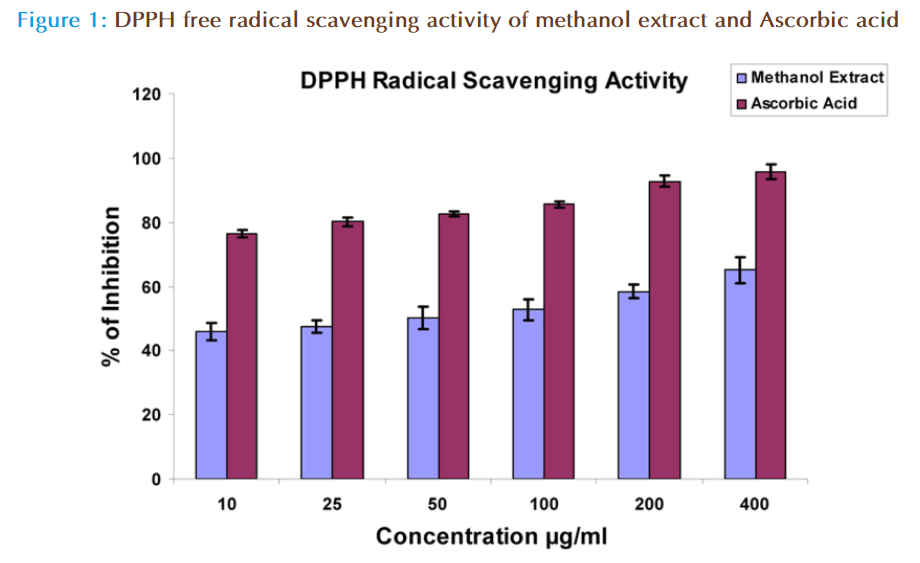
Antioxidant activity of diff erent concentrations of methanol extract of fruit pericarp and ascorbic acid in terms of free radical scavenging ability was evaluated using DPPH free radical assay (Figure 1). The extract exhibited marked antioxidant activity by scavenging DPPH* (free radical) and converting into DPPHH and the activity was found to be dose dependent. The scavenging activity of ascorbic acid was greater than that of methanol extract. The IC50 of the extract and Ascorbic acid was found to 49.42 and 06.09 μg/ml respectively.
The result of anthelmintic activity of methanol extract of fruit pericarp is depicted in Table 2. The extract exhibited marked anthelmintic eff ect by causing paralysis followed by death of worms and the eff ect was found to be dose dependent. The anthelmintic eff ect of extract concentration 50mg/ml was comparable with that of standard drug (1% Piperazine citrate). Anthelmintic eff ect by extract concentration 100mg/ml was higher when compared to that of standard drug as the time taken for causing paralysis and death of worms was shorter.
| Extract/ Drug | Concentration | Paralysis time(in min) | Death time (in min) |
|---|---|---|---|
| Saline | 0.85% | - | - |
| 10 mg/ml | 154 | 188 | |
| 25 mg/ml | 113 | 146 | |
| Methanol extract | |||
| 50 mg/ml | 81 | 113 | |
| 100 mg/ml | 74 | 95 | |
| Standard | 1% | 85 | 114 |
Table 2: Anthelmintic activity of methanol extract
The insecticidal efficacy of diff erent concentrations of methanol extract was evaluated against 2nd and 3rd instar larvae of A. aegypti. The mortality of the larvae was found to be concentration dependent. Among larvae, 2nd instar larvae were shown to be more susceptible than 3rd instar larvae. The highest mortality (85%) of 2nd instar larvae was recorded at concentration 100mg/ml on 3rd day. In case of 3rd instar larvae, highest mortality (50%) was observed on 3rd day with concentration 100mg/ml. Extract concentration 10mg/ml did not caused death of both larval stages even after 72 hours (Table 3).
| Concentration | Mortality Percentage (%) | |||||
|---|---|---|---|---|---|---|
| II Instar Larvae | III Instar Larvae | |||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 10 mg/ml | 0 | 0 | 0 | 0 | 0 | 20 |
| 25 mg/ml | 0 | 40 | 60 | 0 | 20 | 40 |
| 50 mg/ml | 20 | 35 | 70 | 10 | 20 | 45 |
| 100 mg/ml | 50 | 60 | 85 | 25 | 35 | 50 |
Table 3: Insecticidal activity of methanol extract of fruit pericarp
Discussion
Infectious diseases caused by bacteria, fungi, viruses, and parasites remain a major threat to public health, despite tremendous progress in human medicine. Their impact is particularly great in developing countries because of the relative unavailability of medicines and the emergence of widespread drug resistance [15]. Interest in plants with antimicrobial properties has revived as a result of current problems associated with the use of antibiotics [16]. Antimicrobial activities of tannins [17], flavonoids [18], saponins [19], terpenoids [20] and alkaloids [21] have been documented. In this study, the preliminary phytochemical analysis of the methanol extract of fruit pericarp showed the presence of tannins and alkaloids. The antibacterial activity of extract in this study could be chiefly due to the presence of these phytoconstituents and is suggestive of the possible use of the plant in treatment of bacterial infections as most strains have already developed resistance to most of the currently used antibiotics.
Living tissues derive energy from aerobic metabolism and are under constant threat of damage by reactive oxygen derivatives. Such free radicals are usually short-lived species but they possess a single unpaired electron, rendering them highly reactive against biologically important macromolecules including DNA, proteins and membrane lipids. To counteract this threat to their integrity, cells have evolved a variety of defense systems based on both water soluble and lipid-soluble antioxidant species, and on antioxidant enzymes. A high proportion of the antioxidant systems of the human body are dependent on dietary constituents. Consequently, the search for natural antioxidants, especially of plant origin, has notably increased in recent years [22-24]. DPPH radical scavenging is a widely used method to evaluate the free radical scavenging ability of various materials [25]. DPPH is a stable nitrogen-centred free radical, the colour of which changes from violet to yellow upon reduction by either the process of hydrogen- or electron- donation. Substances which are able to perform this reaction can be considered as antioxidants and, therefore, radical scavengers [26]. In this study, it was found that the radical-scavenging activity of methanol extract increased with increasing concentration.
During the past few decades, despite numerous advances made in understanding the mode of transmission and the treatment of these parasites, there are still no efficient products to control certain helminthes and the indiscriminate use of some drugs has generated several cases of resistance. Furthermore, it has been recognized recently that anthelmintic substances having considerable toxicity to human beings are present in foods derived from livestock, posing a serious threat to human health [27]. The origin of many eff ective drugs is found in the traditional medicine practices and in view of this several workers have undertaken studies pertaining to testing of natural compounds for their proclaimed anthelmintic activity. The traditional medicines hold a great promise as a source of easily available eff ective anthelmintic agents to the people, particularly in developing countries, including India [13]. Indigenous system of medicine reports a number of natural sources for their anthelmintic efficacy. However, their scientific evaluation as compared to commercial anthelmintics is limited. Many plants have proven to possess anthelmintic activity in vitro and in vivo. Tannins were found to possess anthelmintic activities. Reported anthelmintic eff ect of tannins is that they can bind to free proteins in the gastrointestinal tract of host animal or glycoprotein on the cuticle of the parasite and may cause death [28-29]. Preliminary phytochemical analysis revealed the presence of tannins in the methanol extract which could be responsible for the anthelmintic eff ect of extract. The result of the present study is suggestive that the extract could be used in the control of round worm infections such as Ascariasis, hookworm infections etc as the worms used in the study are in anatomical and physiological resemblance with the intestinal round worms.
Herbal products with proven potential as insecticide or repellant can play important role in the interruption of the transmission of mosquito borne diseases at the individual as well as community level. Some herbal products have been used as natural insecticides even before the discovery of synthetic organic insecticides [30]. Mosquitoes are the most important single group of insects acting as vector for many tropical and subtropical diseases [31]. The approach to combat these diseases largely relied on interruption of the disease transmission cycle by either targeting the mosquito larvae through spraying of stagnant water breeding sites or by killing the adult mosquitoes using insecticides [32]. Killing larvae of mosquitoes is a successful way of minimizing mosquito densities in breeding grounds before they reach adult stage. It largely depends on the use of synthetic chemical insecticides. But their repeated use has caused environmental problems and widespread development of resistance. Plants off er an alternative source of insectcontrol agents because they contain a range of bioactive chemicals, many of which are selective and have little or no harmful eff ect on non-target organisms and the environment. It is observed that the carbohydrates, saponins, phytosterols, phenols, flavonoids and tannins are having mosquito larvicidal activity [14]. In this study, the larvcidal eff ect of fruit pericarp extract was found to be dose dependent and the eff ect may be due to the presence of phytochemicals present in it. The extract could be used in the prevention of arboviral infections such as dengue, chickungunya etc which are transmitted by A. aegypti.
Conclusion
Plant extracts have been used in the control/treatment of free radical damage, bacterial, helminthic and mosquito borne diseases as the chemical agents have caused some ill eff ects and also the pathogens have developed resistance against them. The results of the present study suggest that the fruit extract selected in this study could be used in treatment of free radical damage, bacterial and helminthic infections and control of arboviral infections transmitted by the mosquito Aedes aegypti. The biological activities of methanolic extract in this study could be chiefly due to the presence of phytoconstituents such as tannins and alkaloids. Further studies on isolation of constituents and determining their in vitro and biological efficacies are under investigation.
Acknowledgement
The authors express their sincere thanks to HOD, Department of Microbiology and Principal, S.R.N.M.N College of Applied Sciences, Shimoga. Authors also thank N.E.S for providing all facilities and moral support to conduct work.
References
- Khare CP. Indian Medicinal Herbs: An illustrated dictionary. Springer- Verlag Berlin/Heidelberg, 2007; pp 66.
- Charoenlarp P, Radomyos P, Harinasuta T. Treatment of taeniasis with Puag-Haad: a crude extract of Artocarpus lakoocha wood. The Southeast Asian Journal of Tropical Medicine and Public Health. 1981; 12: 568-570.
- Salguero CP. A Thai Herbal Traditional Recipes for Health and Harmony. Findhorn Press, Scotland. 2003; pp. 119.
- Joshee N, Bastola DR, Agrawal VP, Yadav, AK. Lakoocha: a multipurpose tree of warm climates. In: Janick J, Whipkey A, eds. Trends in New Crops and New Uses. Alexandria, VA: ASHS Press; 2002.
- Wongkham S, Wongkham C, Boonsiri P, et al. Isolectins from seeds of Artocarpus lakoocha. Phytochemistry. 1995; 40(5): 1331-1334 .
- Puntumchai A, Kittakoop P, Rajviroongit S, et al. Lakoochins A and B, new antimycobacterial stilbene derivatives from Artocarpus lakoocha. J Nat Prod. 2004; 67(3): 485-486.
- Likhitwitayawuid K, Sritularak B, Benchanak K, et al. Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat Prod Res. 2005; 19(2): 177-182
- Manjunatha BK, Patil HSR, Vidya SM, et al. Studies on the antibacterial activity of Mucuna monosperma DC. Indian Drugs 2006; 43: 150-152
- Mathad P, Mety SS. Phytochemical and Antimicrobial activity of Digera Muricata (L.) Mart. E-Journal of Chemistry. 2010; 7(1): 275-280
- Tepe B, Donmez E, Unlu M, et al. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem. 2004; 84(4): 519-525
- Ravikumar YS, Mahadevan KM, Kumaraswamy MN, et al. Antioxidant,Cytotoxic and Genotoxic evaluation of Alcoholic extract of Polyalthia cerasoides (roxb) Bedd. Environ Toxicol Pharmacol. 2008; 26: 142-146
- Grime AS, Bhalke RD, Ghogare PB, et al. Comparative in vitro anthelmintic activity of Mentha piperita and Lantana camara from Western India. Dhaka Univ J Pharm Sci. 2006; 5 (1-2): 5-7.
- Temjenmongla, Yadav AK. Anticestodal efficacy of folklore medicinal plants of Naga tribes in Northeast India. Afr J Trad CAM. 2005; 2(2): 129-133.
- Khanna VG and Kannabiran K. Larvicidal effect of Hemidesmusindicus, Gymnema sylvestre, and Eclipta prostrata against Culex qinquifaciatus mosquito larvae. Afr J Biotech. 2007; 6(3): 307-311.
- Okeke IN, Laxminarayan R and Bhutta ZA. Antimicrobial Resistance in developing countries. Part 1: recent trends and current status. Lancet Infect Dis. 2005; 5: 481-493.
- Abu-Shanab B, Adwan G, Abu-Safiya D. Antibacterial activities of some plant extracts used in Palestine in popular medicine. Turk J Biol. 2004; 28: 99-102.
- Doss A, Mubarack HM, Dhanabalan R. Pharmacological importance of Solanum trilobatum. Ind J Sci Tech. 2009; 2(2): 41-43.
- Mandalari G, Bennett RN, Bisignano G, et al. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J Appl Microb. 2007; 103(6): 2056-2064.
- Avato P, Bucci R, Tava A, et al. Antimicrobial activity of saponins from Medicago sp.: structure-activity relationship. Phytotherapy Res. 2006; 20(6): 454 – 457.
- Funatogawa K, Hayashi S, Shimomura H, et al. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microb Immunol. 2004; 48(4): 251-261.
- Navarro V, Delgado G. Two antimicrobial alkaloids from Bocconia arborea. J Ethnopharmacol. 1999; 66(2): 223-6.
- Nehir ES, Karakaya S. Radical scavenging and iron chelating activities of some greens used as traditional dishes in Mediterranean diet. Int J Food Sci Nutr. 2004; 55(1): 67 -74.
- Nabavi SM, Ebrahimzadeh MA, Nabavi SF, et al. Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacologyonline. 2008; 2: 560-567.
- Ebrahimzadeh MA, Nabavi SM, Nabavi SF, et al. Antioxidant Activity of the Bulb and Aerial Parts of Ornithogalum sintenisii L (Liliaceae) at Flowering Stage. Trop J Pharm Res. 2010; 9 (2): 141-148
- Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Antioxidant activities of methanol extract of Sambucus ebulus L. flower. Pak J Biol Sci. 2009; 12(5): 447-450.
- Dehpour AA, Ebrahimzadeh MA, Nabavi SF, et al. Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites. 2009; 60(4): 405-412.
- Nunomura RCS, Dasilva ECC, Oliverira DF, et al. In vitro studies of the anthelmintic activity of Picrolemma sprucei Hook.f. (Simaroubaceae). Acta Amazonica 2006; 36(3): 327-330.
- Athnasiadou S, Kyriazakis F, Jackson RL, et al. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: In vivo studies. Vet Parasitol. 2001; 99: 19.
- Thompson DP, Geary TG. The structure and fuction of helminth surfaces. In: Marr JJ, Editor. Biochemistry and Molecular biology of Parasites. 1st Edn. New York. Academic press 1995; pp 203-232.
- Mittal PK. Prospects of using herbal products in the control of mosquito vectors. ICMR Bull. 2003; 33(1).
- Service MW. Management of vectors. In: Youdeowei A, Service MW, editors. Pest Vector Management in Tropics, 2nd edn, Longman group Ltd., England. 1983; pp 265-280.
- Joseph CC, Ndoile MM, Malima RC et al. Larvicidal and mosquitocidal extracts, a coumrin, isoflavonoids and pterocarpans from Neorautanenia mitis. Trans R Soc Trop Med Hyg. 2004; 98: 451-455