Review of insulin and its analogues in diabetes mellitus
- *Corresponding Author:
Date of Received: 16-01-2012
Date of Accepted: 20-04-2012
Available Online: 15-05-2012
Abstract
Diabetes is a metabolic disorder where in human body does not produce or properly uses insulin, a hormone that is required to convert sugar, starches and other food into energy. Diabetes finally leads to more complications and to prevent these complications insulin and its analogues are used. After more than half a century of treating diabetics with animal insulin’s, recombinant DNA technologies and advanced protein chemistry made human insulin preparations available in the early 1980s. As the next step, over the last decade, insulin analogues were constructed by changing the structure of the native protein with the goal of improving the therapeutic properties of it, because the pharmacokinetic characteristics of rapid, intermediate and long-acting preparations of human insulin make it almost impossible to achieve sustained normoglycemia. The first clinically available insulin analogue, lispro, confirmed the hopes by showing that improved glycaemic control can be achieved without an increase in hypoglycaemic events. Two new insulin analogues, insulin glargine and insulin aspart, have recently been approved for clinical use in the United States and several other analogues are being intensively tested.
Keywords
Diabetes, Insulin’s, rDNA Technology, Glycaemic,TherapeuticProperty.
Diabetes Mellitus
Diabetes mellitus (DM) is a metabolic disorder resulting from a defect in insulin secretion, insulin action or both [1-4]. Insulin deficiency in turn leads to chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism [1-4]. As the disease progresses tissue or vascular damage ensues leading to severe diabetic complications such as retinopathy [5,6], neuropathy [7,8], nephropa- thy [9,10], Cardiovascular complications [11,12] and ulceration [13,14]. Thus diabetes covers a wide range of heterogeneous diseases. Diabetes is the most common endocrine disorder and by the year 2010, it was esti- mated that more than 200 million people worldwide had DM and 300 million will subsequently have the disease by 2025 [15-17]. The diagnos- tic criteria and the classification of diabetes was first put forward by the World Health Organization (WHO) in 1965[18] then by the National Diabetes Data Group (NDDG) in 1979 [19] and this was followed by simplified recommendations by the WHO in 1980 [20]. These WHO recommendations were modified slightly in 1985 [21]. The latest recom- mendations have been published by the American Diabetes Association (ADA) in 1997 and by the WHO in 1999. Both groups agree on the recommendations and criteria [2, 22].
According to the ADA recommendation changes in 1997, the fasting glucose concentration should be used in routine screening for diabetes as well as epidemiological studies; the threshold for fasting glucose was changed from 7.8 mmol/L (140 mg/dl) to 7.0 mmol/L (126 mg/dl); however the 2 hrs glucose criterion remains as 11.1 mmol/L (200 mg/dL). For the diagnosis of diabetes, at least one of the below criteria must apply.
• Symptoms of diabetes (polyuria, polydipsia, unexplained weight loss, etc) as well as casual plasma glucose concentration = 11.1 mmol/L (200 mg/dL).
• Fasting plasma glucose = 7.0 mmol/L (126 mg/dL), with no caloric intake for at least 8 hrs.
• 2 hrs plasma glucose = 11.1 mmol/L (200 mg/dL) during an oral glucose tolerance test (OGTT), with the glucose load containing 75 g anhydrous glucose in water.
The WHO diagnosis and classification of diabetes mellitus (1999) are identical to those of ADA, a fasting glucose = 7.0 mmol/L (126 mg/dl) and /or a 2 hrs glucose = 11.1 mmol/L (200 mg/dL). The report states that diagnosis should not be based on a single glucose determination but requires confirmatory symptoms or blood/plasma determination. Ideally therefore, both the 2 hrs and fasting value should be used. These recom- mendations contrast with those of ADA Expert Committee which gives primacy to the fasting plasma glucose. The WHO classification includes both clinical stages (normoglycaemia, impaired glucose tolerance/im- paired fasting glucose (IGT/IFG), diabetes and aetiological types of dia- betes mellitus, identical to the ADA except that WHO group includes classification formerly known as gestational impaired glucose tolerance (GIGT) and GDM: fasting glucose = 7.0 mmol/L (126 mg/dL) and/or 2 hrs glucose = 7.8 mmol/ L (140 mg/dL) after a 75-g OGTT.
Diabetes mellitus may be categorized into several types but the two major types are type I and type II [21, 23]. On the basis of aetiology, the term type I and type II were widely used to describe IDDM and NID- DM, respectively. The term juvenile -onset diabetes has sometimes been used for IDDM and maturity-onset for NIDDM.
Type I (Ia, Ib) ß-cell destruction with little or no endogenous insulin secretory capacity Autoimmune Idiopathic Type II Ranges from relative insulin deficiency to disorders of insulin secretion and insulin resistance.
On the basis of etiology, type I is present in patients who have little or no endogenous insulin secretory capacity and who therefore require insulin therapy for survival. The two main forms of clinical type I dia- betes are type Ia (about 90% of type I cases in Europe) which is thought to be due to immunological destruction of pancreatic ß cells resulting in insulin deficiency, and type Ib (idiopathic, about 10% of type I diabetes), in which there is no evidence of autoimmunity. Type Ia is characterized by the presence of islet cell antibody (ICA), anti-glutamic acid decar- boxylate (anti-GAD), Ia-2 or insulin antibodies that identify the autoim- mune process with ß-cell destruction.[23,24] Autoimmune diseases such as Grave’s disease, Hashimoto’s thyroiditis and Addison’s disease may be associated with type I diabetes mellitus [24,25]. There is no known etiological basis for type Ib diabetes mellitus. Some of these patients have permanent insulinopaenia and are prone to ketoacidosis but, have no evi- dence of autoimmunity [26]. This form is more prevalent among individ- uals of African and Asian Origin [27]. Type II diabetes is the commonest form of diabetes and is characterized by disorders of insulin secretion and insulin resistance [28]. In Western countries the disease affects up to 7% of the population [29,30]. Globally, it affects 5-7% of the world’s popu- lation [15, 16, 30]. However this prevalence is underestimated because many cases, perhaps 50% in some population, remain undiagnosed. The prevalence of type II diabetes varies considerably throughout the world, ranging from <1% in certain population of the developing countries for example rural Melanesians in Papua New Guinea and rural Chinese, to over 50% in the Pima Indians of Arizona [31]. There is a higher inci- dence of type II diabetes in urban than in rural areas [16, 31, 32]. Its incidence is associated with population whose lifestyle has changed from traditional patterns to a modern ‘‘Westernized’’ model [33]. The classical example includes the Pima Indians, Chinese who moved to Mauritius and Japanese who immigrated to Hawaii [29, 33-35]. Traditionally, type II diabetes is common in individuals over the age of 40. It is often as- sociated with obesity, decreased physical activity and heredity [36, 37]. Recent data from several countries show that type II diabetes is increas- ingly becoming a problem among adolescents and even children [38, 39]. In some countries, childhood diabetes type II is more common than type I [40]. The disease is usually controlled through dietary therapy, exercise and hypoglycaemic agents [41, 42].
Symptoms
Symptoms are similar in both types of diabetes but they vary in their intensity. Symptoms develop more rapidly in type I diabetes and more typical. The symptoms include polyuria, polydipsia, polyphagia, weight loss, fatigue, cramps, constipation, blurred vision, and candidiasis [1]. Longstanding type I DM patients are susceptible to microvascular complications [5-10] and macrovascular disease (coronary artery, heart, and peripheral vascular diseases) [11, 12]. Symptoms in type II DM are similar but insidious in onset. Most cases are diagnosed be- cause of complications or incidentally. Type II DM carries a high risk of large vessel atherosclerosis commonly associated with hypertension, hyperlipidaemia and obesity [11, 12, 36, 37]. Most patients with type II diabetes die from cardiovascular complications and end stage renal disease [9-12]. A geographical difference exists in both the magnitude of these problems and their relative contributions to overall morbidity and mortality [34, 35].
Insulin
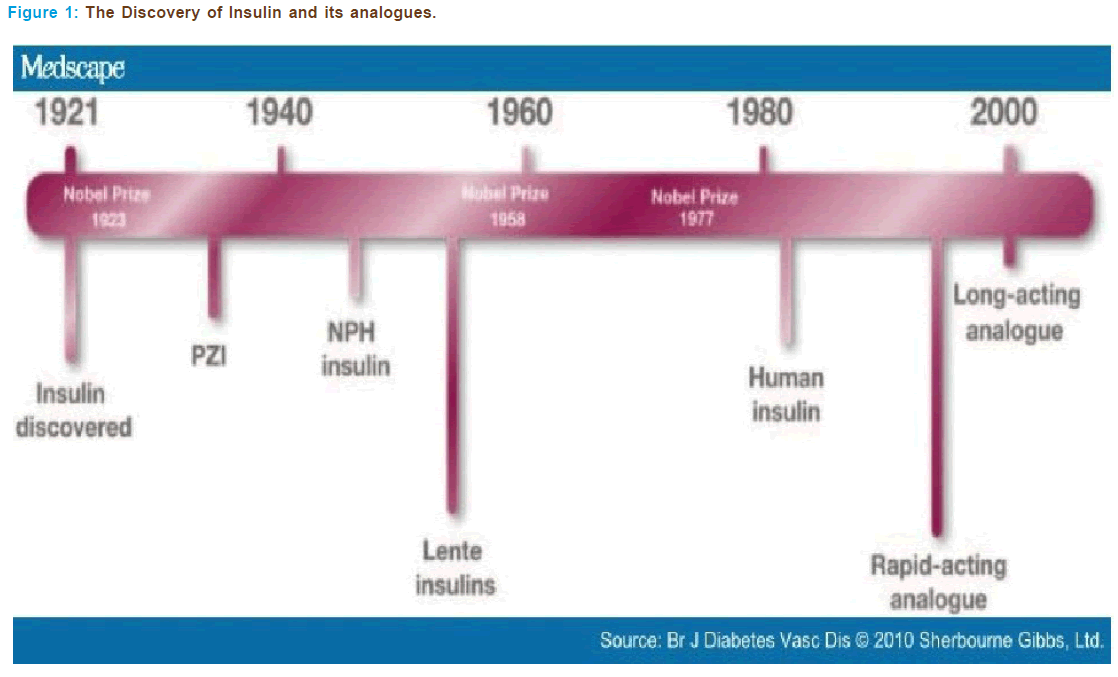
Compliance with the insulin therapy is important in preventing the ad- verse clinical effects of the disease. Insulin treatment in type I and type II diabetes has come a long way since its discovery by Banting and Best in 1922 [55]. In human beings the β-cells of pancreatic islets of Lang rhans synthesize insulin from a single-chain precursor of 110 amino acids termed preproinsulin. Insulin was purified and crystallized by Abel with- in a few years of its discovery. Sanger established the amino acid sequence of insulin in 1960 and it was synthesized in 1963. However Hodgkin and co-workers have elucidated insulin’s three-dimensional structure in 1972 [56, 57].
In 1980s with the help of recombinant DNA technology, human in- sulin were discovered which replaced animal insulin’s (Figure 1). With the advent of high-pressure liquid chromatographic technique, the level of purification of animal-sourced insulin’s has reached as high as 99%, whereas the purity level of synthetic human insulin’s made via recom- binant DNA has only attained a maximum purity level of 97%, which raises questions about the claim of synthetic insulin’s purity related to animal-sourced insulin varieties Human insulin’s have reduced the ad- verse effects of animal insulin’s such as insulin allergy, insulin resistance and insulin lipodisatrophy[ 56, 57].
Structure and Chemistry
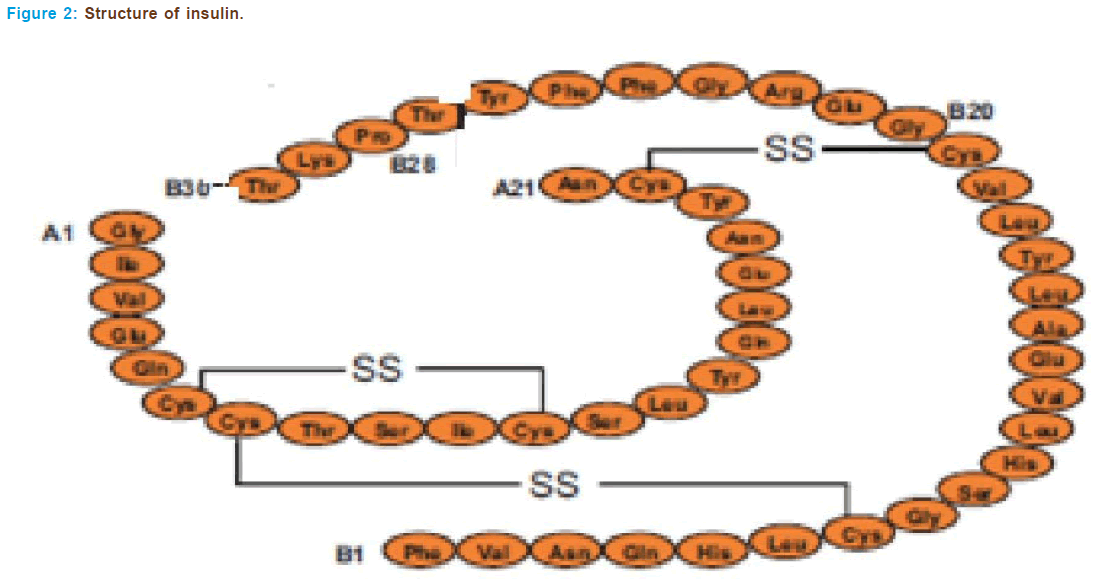
The insulin gene is a protein consisting of two separate chains of amino acids, an A and B chain, that are held together with sulphide bonds. Amino acids are the basic units that build all proteins. The insulin A chain consists of 21 amino acids and the B chain has 30 (Figure 2).
The βcells of pancreatic islets synthesize insulin from a single-chain precursor of 110 amino acids termed preproinsulin. After translocation through the membrane of the rough endoplasmic reticulum, the 24- amino-acid N-terminal signal peptide of preproinsulin is cleaved rapidly to form proinsulin. Thereafter, proinsulin folds and the disulfide bonds form. During conversion of human proinsulin to insulin, four basic amino acids and the remaining connector or C peptide are removed by proteolysis. This gives rise to the A and B peptide chains of the insulin molecule, which contains one intrasubunit and two intersubunit disulfide bonds. The A chain usually is composed of 21 amino acid residues and the B chain has 30, the molecular mass is thus about 5808 daltons. There is a single insulin gene and a single protein product in most species. How- ever, rats and mice have two genes that encode insulin and synthesize two molecules that differ at two amino acid residues in the B chain.
The crystal structure reveals that the two chains of insulin form a highly ordered structure with helical regions in each of the chains. The isolated chains of insulin are inactive. In solution insulin can exist as a monomer, dimer or hexamer. Two molecules of Zn2+ are coordinated in the hexamer, and this form of insulin presumably is stored in the gran- ules of the pancreatic cell. It is believed that Zn2+ has a functional role in the hexamer formation and that this process facilitates the conversion of proinsulin to insulin and storage of the hormone. Traditional insulin is hexameric in most of the highly concentrated preparations used for therapy. When the hormone is absorbed and the concentration falls to physiological levels (nanomolar), the hormone dissociates into mono- mers and the monomer is most likely the biologically active form of insu- lin. Monomeric insulin is now available for therapy.
Substantial information about the structure-activity relationship of insulin has been obtained by study of insulin’s purified from a wide vari- ety of species and by modification of the molecule. A dozen invariant resi- dues in the A and B chains form a surface that interacts with the insulin receptor. These residues-GlyA1, GluA4, GlnA5, TyrA19, AsnAA21, ValB12, TyrB16, GlyB23, PheB24, PheB25, and TyrB26-overlap with domains that also are involved in insulin dimerization. The LeuA13 and LeuB17 residues may form part of a second binding surface. Insulin binds to surfaces lo- cated at the N- and C-terminal regions of the subunit of the receptor, including a cysteine-rich region in the receptor chain. In most cases, the affinity of insulin for the insulin receptor correlates closely with its po- tency for eliciting effects on glucose metabolism. However human, bovine and porcine insulin’s are equipotent [56, 57].
Insulin Analogues
In no diabetic individuals, ingestion of food results in a relatively rapid rise of serum insulin concentration to a maximum after 30-45 min followed by a decline to basal levels after 2-3 hrs. The pharmacokinetic characteristics of the currently available rapid, intermediate and long- acting preparations of human insulin make it almost impossible to achieve sustained normoglycemia. The onset of action of SC-injected regular human insulin is too slow and the duration of its action too long to mimic the insulin secretion pattern of a healthy individual dur- ing ingestion of a carbohydrate- containing meal [58]. As a result, early postprandial hyperglycaemia followed by an increased risk for hypoglycaemia before the next meal are present. Similarly the available inter- mediate/long-acting human insulin preparations are unable to provide a stable, continuous baseline insulin level. Instead they cause peak serum insulin levels at 3-4 hrs after SC injection and show considerable inter- and intrasubject variations in their bioavailability. The Diabetes Control and Complications Trial confirmed the link between glycaemic control and the complications of diabetes [59]. Therefore, to achieve improved glucose control, the need for new insulin preparations with a faster onset and shorter duration of action and for long-acting preparations with a more flat time-action profile and less variable bioavailability became ap- parent in the late 1980s and early 1990s [60]. However, until recently, improvements in insulin formulations were seriously limited; advances were only achieved in insulin purity, species and characteristics of the retarding agent. The availability of molecular genetic techniques opened new windows for creating insulin analogues by changing the structure of the native protein, improving its therapeutic properties. In addition to its glucose lowering effect, insulin is the most potent physiological anabolic agent known to date [61]. It promotes the synthesis and stor- age of lipids, proteins and carbohydrates and prevents their degradation and release back to the circulation. Despite years of intensive investiga- tion, we are still left with considerable uncertainty regarding the precise intracellular events that mediate the action of this hormone. One con- founding factor has been the variety of actions of insulin, which depend on the cell type, time of exposure, and the presence or absence of other hormones [62]. Another is the fact that insulin can act as a growth factor for cultured cells and shares many of the mitogenic signalling pathways elicited by other growth factors. However, the metabolic effects of insulin are unique and cannot be reproduced by other cellular stimuli [60, 63]. Taken together, these findings indicate that signalling mechanisms that respond only to insulin exist, and they allow for the specialized ef- fects of insulin on metabolism. Designing and studying insulin analogues has helped and without any doubt will help, our understanding of the complex processes insulin is associated with and creating analogues se- lective to one or another of insulin’s actions might well be of clinical significance.
Insulin lispro (Eli Lilly & Co., Indianapolis, IN)
Scientific Information
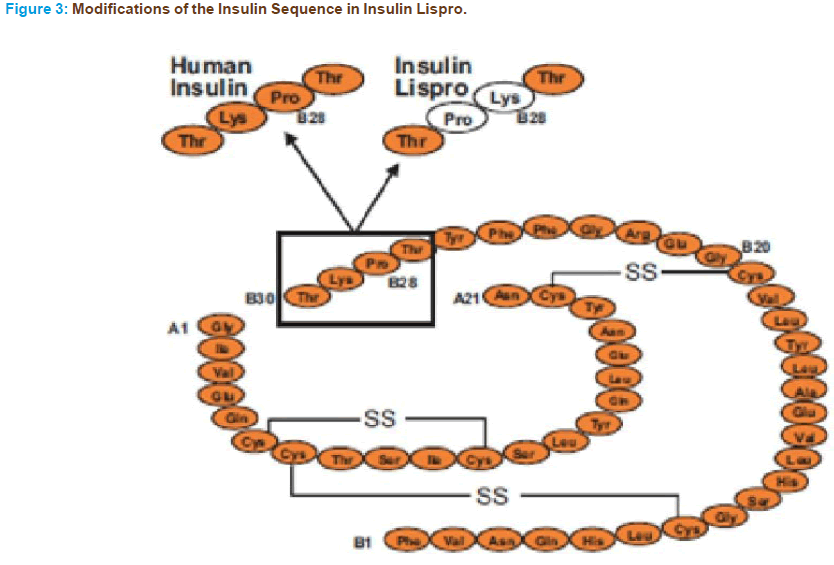
• Chemical Name: Lys(B28),Pro(B29) -human insulin
• Molecular weight: 5808 Dalton’s
• Molecular Formula: C257H383N65S6 O77
The B26 -30 region of the insulin molecule is not critical in binding to the insulin receptor. However, it is clearly important in mediating the formation of insulin dimers [66]. Therefore structural modifications of the molecule at these positions would be expected to generate insulin analogs with minimal tendency for self-association but unaltered affinity to the in- sulin receptor compared with regular human insulin [65]. The first geneti- cally engineered rapid-acting insulin analogue to become available for the clinician was insulin lispro, which was approved for clinical use in Europe in April of 1996 and in the United States in June of 1996. In insulin lispro, the normal sequence of proline at position 28 of the B chain and lysine at position 29 is reversed (Figure 3). This reversal causes a decreased ten- dency for self-association and as a result faster absorption, higher peak serum levels and shorter duration of action can be observed with insulin lispro compared with regular insulin. Importantly, as discussed above, the amino acid sequence changes in lispro do not affect its receptor-binding domain. Therefore the affinity to the insulin receptor of insulin lispro is similar to that of regular insulin. Although lispro affinity for the IGF-I receptor is slightly higher, it is not enough to cause a difference in its cell growth-stimulating activity compared with regular insulin [68, 69].
In terms of activity on lipogenesis, insulin lispro was found to be es- sentially the same as human insulin [64]. Pharmacokinetic studies in- dicate that insulin lispro acts within 15 min, peaks in approximately 1 hrs and disappears within 2-4 hrs after SC injection [67, 70]. In clinical studies, as expected from a short-acting analogue, insulin lispro achieved significant improvements in postprandial glucose levels with a lower rate of hypoglycaemic events compared with regular insulin [71-73]. This can be observed even if insulin lispro is administered immediately before meals and regular insulin is injected 30-45 min before meals. Unfortu- nately, in most cases these beneficial effects were not accompanied by im- provements in glycosylated haemoglobin values [71, 72]. In addition to the decrease in hypoglycaemic events, the most likely explanation for this is the inability of the currently used long-acting insulin’s to provide true basal coverage. Therefore, increased pre-prandial plasma glucose concen- trations are present in patients on insulin lispro. Supporting this theory, a clinically and statistically significant decrease of hemoglobinA1c levels was seen when insulin lispro was used with two or more daily injections, instead of one, of neutral protamine Hagendorn (NPH) insulin [74, 75]. Therefore, for the intensive therapy of diabetes by multiple daily injec- tions, the addition of a few units of NPH to lispro at each meal, com- bined with bedtime NPH, can be recommended [75-77]. This regimen may even improve unawareness of and impaired counter regulation to hy- poglycaemia [77]. Insulin lispro has also been tested for use in pregnancy and gestational diabetes [78, 79].
Based on the limited available data on its long-term effectiveness, it ap- pears that insulin lispro remains effective in treating diabetic patients up to 5.4 years of treatment [80]. No differences have been reported between insulin lispro and regular insulin in the likelihood of developing allergic reactions, adverse events or abnormal laboratory values [81]. The immunogenicity of insulin lispro is similar to that of regular insulin [82]. Antibod- ies specific against insulin lispro hardly ever develop and do not affect dose requirements [80, 83]. Interestingly, there have been reports of patients in whom severe resistance to human insulin due to antibody formation was successfully overcome by switching them to insulin lispro [84, 85].
Insulin Glargine [HOE 901, LANTUS (Aventis Pharmaceuti- cals, Parsippany, NJ)]
Insulin glargine is a recombinant human insulin analogue produced by DNA technology using non-pathogenic strains of Escherichia coli, Pichia pastoris.
HOE 901 (insulin glargine, LANTUS) is a new long-acting biosyn- thetic human insulin analogue developed by Aventis Pharmaceuticals, which was approved for use in patients with type I and type II diabetes mellitus by the United States Food and Drug Administration in April of 2000 and by the European Agency for the Evaluation of Medicinal Products in June of 2000 [86,87].
Scientific Information
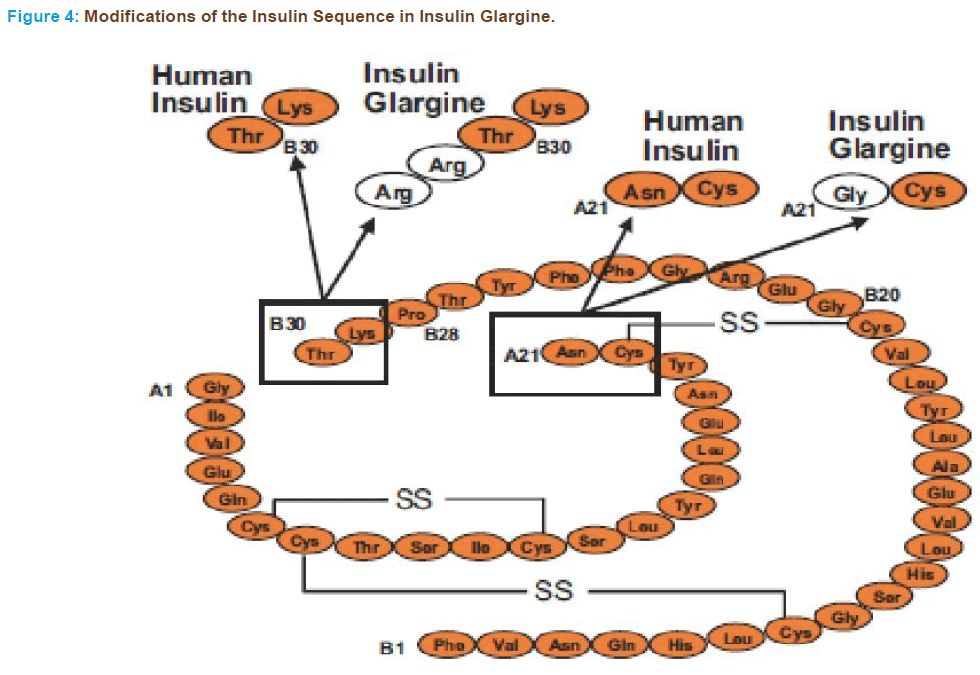
• Chemical Name: 21A-Gly-30Ba-L-Arg-30Bb-L-Arg-human insulin
• Molecular weight: 6063 Dalton’s
• Molecular Formula: C267H404N72S6 O78
Two modifications of human insulin result in a stable molecule which is soluble in slightly acidic conditions (pH 4.0) and precipitates in the neutral pH of subcutaneous tissue. Because of these properties, absorp- tion of insulin glargine is delayed and the analogue provides a fairly constant, basal insulin supply without peaks in plasma insulin levels for approximately 24 hrs, similar to that achieved by a continuous subcuta- neous insulin infusion [86, 87].
The structure was designed by substituting an asparagine residue with a glycine at position 21 of the A-chain and elongating the B-chain at the C-terminus by addition of 2 arginine residues (Figure 4). Modification of B-chain caused the pH to shift from 5.4 to 6.7 and makes it less soluble at physiological pH and more soluble at acidic pH. The glycine substitu- tion of A chain of insulin glargine stabilizes the hexamer structure and therefore, contributing to delayed delivery from subcutaneous depot and maintaining its stability in acidic solution. Insulin glargine is not to be mixed with other insulin, as it becomes cloudy and results in alteration of pharmacokinetic and pharmacodynamics profile. It precipitates at physi- ological pH and absorbs slowly from injection site.
After SC injection insulin glargine precipitates in the SC tissues, which delays its absorption and prolongs its duration of action [88]. The substitution at position A21 largely increased the bioavailability of this analogue, so unlike Novo Sol Basal, it is suitable for clinical use [89]. With respect to insulin receptor binding, receptor auto phosphorylation, phosphorylation of signalling elements, and promotion of mitogenesis in muscle cells, insulin glargine behaves like regular human insulin [90]. Moreover, the growth-promoting activity of HOE 901 in muscle cells and the maximal metabolic activity of this analogue are not different from those of native human insulin; whereas its lipogenic activity is slightly lower [91]. However, insulin glargine therapeutic properties and poten- tials are remarkable and different from human insulin. HOE 901 was shown to exert a glucose-lowering effect for 24 hrs after a single daily injection without a pronounced plasma peak and induced a smoother metabolic effect than NPH insulin [88, 92]. Thus HOE 901 is expected to better substitute basal insulin requirements. Moreover, although it is well known from clinical practice that the effect of NPH insulin can vary with the site of injection, it has been found that changes in the injection site do not alter the time-action profile of HOE 901[93, 94]. In one of the first small, short-term clinical studies investigating this analogue in 1996, once-daily injections of HOE 901 resulted in similar glycaemic control as compared with four daily injections of the same total units of NPH in type I diabetics [95]. The characteristics of HOE 901 have been investigated in both type I and type II diabetic patients. In phase II trials conducted in Europe and the United States with type I diabet- ics, once-daily injections of HOE 901 along with premeal regular insulin achieved significantly lower fasting plasma glucose levels [96] and hemo- globinA1c values compared with patients on NPH and regular insulin [97]. Remarkably, the better glucose control was associated with simi- lar or even lower incidences of hypoglycaemia. Studies of type 2 diabetic subjects showed similar fasting plasma glucose values with one injection of HOE 901 compared with those found with one or two injections of NPH insulin. Again, the incidence of hypoglycaemia was similar or lower among patients on HOE 901[98-100]. More recently, the findings of less frequent hypoglycaemic episodes and lower fasting plasma glucose levels compared with NPH were confirmed in large, multicentre clinical trials with type I and type II diabetics in Europe and the United States [101- 104]. Considering that less hypoglycaemia was consistently observed, these data suggest that the target fasting plasma glucose level can be lower for insulin glargine than for NPH [104]. The technical difficulties with blinding the studies comparing NPH and HOE 901 should be noted, as the two preparations can be easily identified because HOE 901 is a clear solution as opposed to the cloudy solution of NPH. It might make designing blinded research studies more difficult, but in daily clinical life it could actually be an advantage that insulin glargine is a clear solution. It has been shown that patients do not sufficiently shake suspensions like NPH insulin before administration [105]. Because it is not necessary to shake HOE 901 before usage it may have a lower intraindividual vari- ability of its metabolic effect. In recent clinical trials patients treated with insulin glargine had less variability of their fasting plasma glucose values than those receiving.
Insulin Aspart [Novo Log (Novo Nordisk, Princeton, NJ)
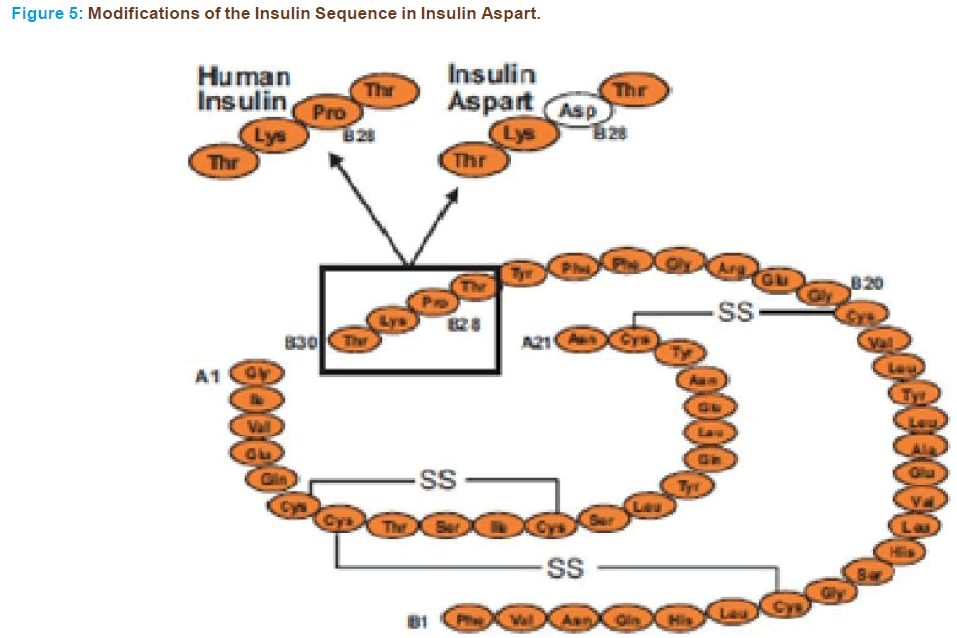
In insulin aspart, substitution of proline (Figure 5) with the charged aspartic acid is carried out to reduce self-association of the molecule [106]. Like lispro it is a short acting analogue. Novo Nordisk created ‘‘aspart’’ and marketed it as Novo Log/Novo Rapid (UK-CAN) as a rapid acting insulin analogue. It was created through recombinant DNA technology so that the amino acid B28, which is normally proline, is substituted with an aspartic acid residue. The sequence was inserted into the yeast genome and the yeast expressed the insulin analogue, which was then harvested from a bioreactor. This analogue also pre- vents the formation of hexamer to create faster acting insulin. This ana- logue was approved for clinical use in the United States in June of 2000. Preclinical studies of insulin aspart have demonstrated that receptor in- teraction kinetics with the insulin receptor and with the IGF-I receptor are essentially equivalent to those seen with human insulin [107] and an equivalent metabolic effect of insulin aspart and human insulin has been shown with iv administration [108]. The potency on lipogenesis of insulin aspart is similar to that on human insulin whereas its affinity to the IGF-I receptor is slightly lower and thus it does not result in greater mitogenic potency [65]. When administered IV insulin aspart shows a similar safety profile with that of human insulin [109]. When further assessing its safety it was found that insulin aspart and soluble human insulin elicit the same counter regulatory and symptomatic re- sponses to acute hypoglycaemia in patients with type I diabetes [110]. Insulin aspart has been shown to be absorbed twice as fast as human insulin and to reach maximum concentrations twice as high whereas its duration of action is shorter [111-113]. As expected, the postprandial glucose control achieved with this analogue is superior to regular hu- man insulin, whereas their bioavailability is comparable [112]. Mean postprandial glucose levels after any meal are lower, even when aspart is injected immediately before the meal and regular human insulin is administered 30 min before meals [114]. These results are consistent with those reported with the other short-acting analogue lispro but there is evidence that the improvement in postprandial control can be achieved without deterioration of late postprandial plasma glucose concentrations [115]. The expectation of lower rates of hypoglycaemia also seems to have been met with insulin aspart, as evidenced by a re- cent multicentre trial of type I diabetic patients, which showed more than a 50% reduction in major hypoglycaemic events compared with human insulin [115]. In a very interesting study with type I diabetics, it was found that, because of its rapid absorption, insulin aspart provided reasonable glucose control even when injected 15 min after the start of meals [116]. In the same study it was also found that after abdominal injections, aspart had a shorter duration of glucose lowering effect than after administration in the thigh or deltoid area [116]. The beneficial effects of insulin aspart have also been confirmed in type II diabetics [117] and in a paediatric population with type I diabetes [118]. Impor- tantly this analogue retains its beneficial pharmacodynamics proper- ties in a stable 30/70 premixed formulation, as it shows a significantly greater metabolic effect in the first 4 hrs with more rapid absorption and higher peak serum concentration than the 30/70 mixture of hu- man insulin [119,120]. Because of its promising characteristics, studies are presently underway to evaluate long-term metabolic control with insulin aspart.
Insulin Detemir
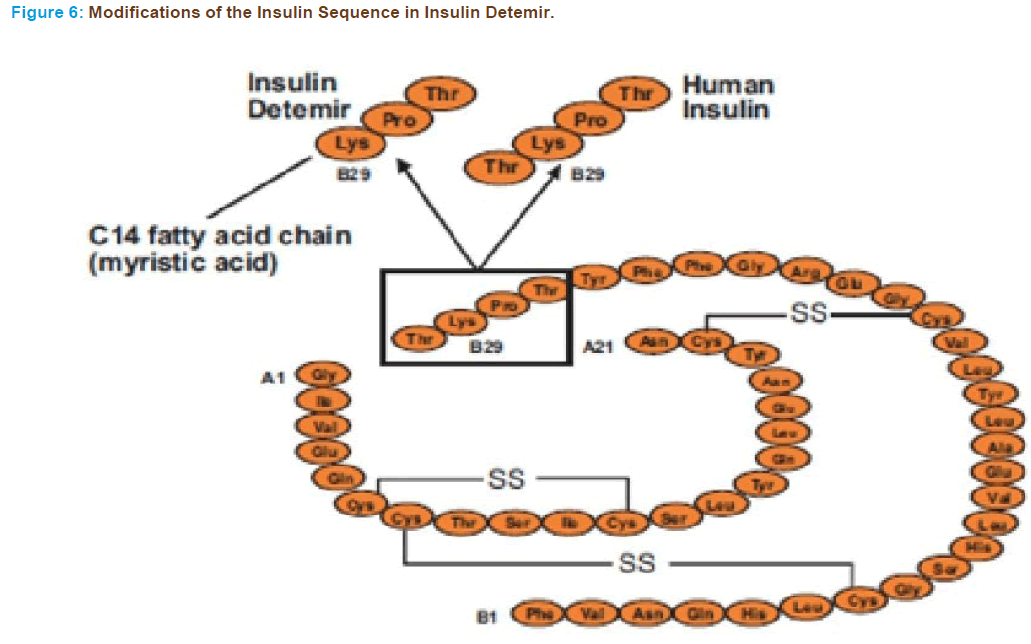
In insulin detemir a C14 fatty acid side chain is attached to lysine at po- sition B29 (Figure 6) in the molecule by an acylation reaction. It is an intermediate acting insulin analogue [121].
Novo Nordisk created insulin detemir and markets it under the trade name Levemir as a long-lasting insulin analogue for maintaining the basal level of insulin. The basal level of insulin may be maintained up to 20 hrs, but the time is clearly affected by the size of the injected dose. This insulin has a high affinity for serum albumin, increasing its duration of action [122, 123].
Glulisine Insulin
Glulisine is a newer rapid acting insulin analogue and the FDA-approved label states that it differs from regular human insulin by its rapid onset and shorter duration of action [56, 57].
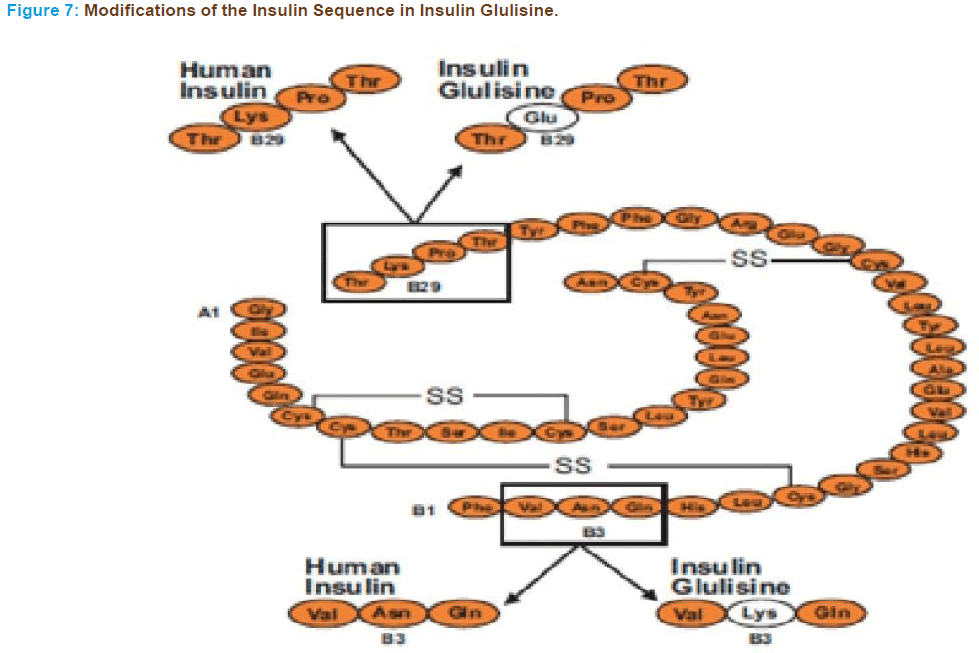
In insulin glulisine the natural sequence of asparagine at position B3 and lysine at position B29 are substituted by lysine and glutamic acid re- spectively (Figure 7) [125, 126]. This structure of insulin glulisine affects not only self-association but also the isoelectric point, which is shifted lower (pH 5.1; human insulin, pH 5.5), which enhances its solubility at a physiologic pH. As a consequence unlike other insulin analogues that lack proline at B28, insulin glulisine is more likely to self-associate into dimers in the absence of ligands.
Conclusion
Diabetes mellitus is a metabolic disorder occurs due to insulin deficiency. The disease may leads to various metabolic complications and to treat these, r-insulin and its analogues are used.
In early years human insulin preparations are replaced by animal insulin’s due to recombinant DNA technologies and advanced protein chemistry. Over the last decade, a number of insulin analogs were de- veloped and to tested in the therapy of diabetes. The insulin lispro was one such short acting insulin anolog used clinically for the first time. This showed the improved glycemic control without an increase in hypoglyc- emic events. Later the availability of long-acting analogs such as insulin glragine replaced to short-acting analog insulin lispro. This helped to de- velop more individualized treatment strategies targeted to specific patient characteristics and to achieve further improvements in glycemic control. Combining different insulin analogs may even help to treat the multi- ple metabolic abnormalities of diabetics which are even beyond glucose metabolism.
References
- Kumar PJ, Clark M, Textbook of Clinical Medicine. Pub: Saunders (London). 2002; pp. 1099-21.
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183-97.
- Beverley B, Eschwège E. The diagnosis and classification of diabetes and im- paired glucose tolerance. In: Textbook of Diabetes 1 Ed: John C Pickup and Gareth Williams Third edition; Chapter 2. 2003; pp. 2.1-2.1
- Lindberg G, Lindblad U, Melander A. Sulfonylureas for treating type II diabetes mellitus. Cochrane Database Systemic Reviews. 2004; 3.
- Bearse MA J et al., Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Ophthal Vis Sci. 2004; 45: 3259-65.
- Hove MN, Kristensen JK, Lauritzen T, Bek T, the prevalence of retinopathy in an unselected population of type II diabetes patients from Arhus County, Denmark. Acta Ophthalmol Scand. 2004; 82: 443-48.
- Seki M et al., Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factors for dopaminergic amacrine cells. Dia- betes 2004; 53: 2412-19.
- Moran A et al., Cardiovascular autonomic neuropathy is associated with micro albuminuria in older patients with type II diabetes. Diabetes care 2004; 27: 972-77.
- Huang C et al., Cellular basis of diabetic nephropathy: II. The transforming growth factor-beta system and diabetic nephropathy lesions in type I diabetes. Diabetes 2002; 51: 3577-81.
- Shukla N et al., Homocysteine as a risk factor for nephropathy and retinopa- thy in type II diabetes. Diabetologia. 2003; 46; 766-72.
- Svensson M, Eriksson JW, Dahlquist G. Early glycaemic control, age at onset, and development of microvascular complications in childhood-onset type I diabetes: a population-based study in northern Sweden. Diabetes Care 2004; 27: 955-62.
- Saely CH et al., Cardiovascular complications in type II diabetes mellitus de- pend on the coronary angiographic state rather than on the diabetes state. Diabetologia. 2004; 47: 145-46.
- Wallace C et al., Incidence of falls, risk factors for falls and fall-related fac- tures in individuals with diabetes and a prior foot ulcer. Diabetes Care 2002; 25: 1983-86.
- Centers for Disease Control and Prevention (CDCP). History of foot ulcer among persons with diabetes Unites States, 2000-2002. Morbidity & Mortality Weekly Report. 2003; 52: 1098-02.
- Amos A, McCarty D, Zimmet P. The rising global burden of diabetes and its complications, estimates and projections to the year 2010. Diabetic Med 1997; 14: S1-S85.
- King H, Aubert R, Herman W. Global burden of, 1995-2025. Prevalence, nu- merical estimates and projections. Diabetes Care 1998; 21: 1414-31.
- Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted? J Med 2000; 247: 301-10.
- World Health Organization Expert Committee on Diabetes Mellitus. Second WHO Technical Report, Series 310. Geneva: World Health Organization, 1965.
- National Diabetes Data Group. Classification and diagnosis of diabetes mel- litus and other categories of glucose intolerance. Diabetes 1979; 18: 1039-57.
- World Health Organization Expert Committee on Diabetes Mellitus. Second WHO Technical Report, Series 646 Geneva: World Health Organization 1980.
- World Health Organization Study Group. Diabetes Mellitus: WHO Technical Report, Series 727. Geneva: World Health Organization, 1985.
- World Health Organization Consultation. Definition, Diagnosis and Classifica- tion of Diabetes Mellitus and its Complications, Part 1: Diagnosis and classi- fication of Diabetes Mellitus. Report of a WHO Consultation. Geneva: World Health Organization, 1999.
- Zimmet P, Cowie C, Ekoe JM, Shaw JE. Classification of diabetes mellitus and other categories of glucose intolerance. In: International Textbook of Diabe- tes Mellitus chapter 1, 3rd Ed, 2004; pg. 3-14.
- Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med 1994; 331: 1428-36.
- Betterle C, Zannette F, Pedini B, et al. Clinical and subclinical organ-specific autoimmune manifestations in type I (insulin-dependent) diabetic patients and their first-degree relatives. Diabetologia 1983; 26: 431-36.
- McLarty DG, Athaide I, Bottazzo GF, et al. Islet cell antibodies are not specifi- cally associated with insulindependent diabetes in rural Tanzanian Africans. Diabetes Res Clin Pract 1990; 9: 219-24.
- Ahrén B, Corrigan CB. Intermittent need for insulin in a subgroup of diabetic patients in Tanzania. Diabet Med 1984; 2: 262-64.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. In Albert KGMM, Zimmet P, DeFronzo RA (Eds) International Textbook of Diabetes Mellitus, 2nd edn. Chichester: Wiley, 1997; pp. 635-12.
- Report of World Health Organization Study Group. WHO Technical Report Series 1994; World Health Organization, 844: Geneva, Prevention of Diabetes Mellitus.
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS. Goldstein DE. Little RR, Wied- meyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: the third National Health and Nutritional Examination Survey, 1988-1994. Diabetes Care, 1998; 21: 518-24.
- Zimmet P. Type 2 (non-insulin -dependent) diabetes: an epidemiological over- view. Diabetologia. 1982; 22:399- 11.
- Christacopoulos PD et al., The prevalence of diabetes mellitus and non-diabet- ic glycosuria in a rural population in Greece. Diabetologia. 1975; 11: A-335.
- Bloomgarden ZT. American Diabetes Association annual meeting 1996: the etiology of type II diabetes, Obesity, and the treatment of type II diabetes. [Congresses] Diabetes Care. 1996; 19: 1311-15.
- Sekikawa A et al., Prevalence of diabetes and impaired glucose tolerance in Funagata area in Japan. Diabetes Care. 1993; 16: 57- 74.
- Ramachandran A et al. Rising prevalence of NIDDM in an urban population in India. Diabetologia. 1997; 40: 232-37.
- Zimmet P, Dowse G, Finch C, Serjeantson S, King H. The epidemiology and natural history of NIDDM - lessons from the South Pacific. Diabetes Metab Rev. 1990; 6: 91-124.
- Eriksson J, Saloranta C, Widen E, et al. Non-esterified fatty acids do not con- tribute to insulin resistance in persons at increased risk of developing type 2 (noninsulin- dependent) diabetes mellitus. [Journal Article] Diabetologia. 1991; 34:192-97.
- Glaser NS. Non-insulin-dependent diabetes mellitus in childhood and adolescence. Pediatr Clin North Am 1997; 44: 307-37
- Scott CR, Smith JM, Pihoker C. Characteristics of youth-onset non-insulin- dependent diabetes mellitus and insulin-dependent diabetes mellitus at diag- nosis. Pediatr 1997; 100: 84-91.
- Cockram CS. Diabetes mellitus: perspective from the Asia-Pacific region. Diab Res Clin Pract 2000; 50 (suppl 2): S3-S7.
- Zimmet P, Alberti KGMM, Shaw T. Global and Social implications of diabetes epidemic. Nature 2001; 414: 782-87.
- Miller CD et al., Hypoglycaemia in patients with type 2 diabetes mellitus. Arch Intern Med; 2001; 161: 1653-59.
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2001; 24 (suppl 1): S77-S79.
- Raffel LJ, Scheuner MT, Rotter JI. Genetics diabetes. In: Porte D Jr, Sherwin RS, eds. Ellenberg & Rifkin’s Diabetes Mellitus, 5th ed. Stamford, CT, Apple- ton & Lange, 1997; 401-54.
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2001; 24 (suppl 1): S5-S19.
- Pandit Mk, Burke J, Gustafson AB. Drug-induced disorders of glucose toler- ance. Ann Intern Med 1993; 118:529-39.
- Froguel P et al., Close linkage of glucokinase locus on chromosomes 7p to ear- ly-onset non-insulin-dependent diabetes mellitus. Nature 1992; 356: 162-64.
- Bell GI et al., Gene for noninsulin- dependent diabetes mellitus (maturity-on- set diabetes of the young subtype) is linked to DNA polymorphism on human chromosome 20q. Proc Natl Acad Sci USA 1991; 88: 1484-88.
- Vaxillaire M et al., A gene for maturity onset diabetes of the young (MODY) maps to chromosome 12q. Nature Genet 1995; 9: 418-23.
- Horikawa Y et al., Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nature Genet 1997; 17: 384- 85.
- Malecki MT et al., Mutations in NEUROD1 are associated with the develop- ment of type II diabetes mellitus. Nature Genet 1999; 23: 323- 28.
- Lederman HM. Is maturity onset diabetes at young age (MODY) more com- mon in Europe than previously assumed? Lancet 1995; 345: 48.
- O’Dea K. Marked improvement in carbohydrate and lipid metabolism in dia- betic Australian Aborigines after temporary reversion to traditional lifestyle. Diabetes 1984; 33: 596-03.
- Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 11th Edition.
- Wilson and Griswold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry 11th Edition. Lippincott Williamsons and Wilkins, Philadelphia. New York. 2004.
- Heinemann L, Starke AAR, Hohmann A, Berger M. Timing between the subcutaneous administration of insulin and consumption of a carbohydrate rich meal. Horm Metab Res Suppl. 1992; 26:137–39.
- Diabetes Control and Complications Trial Research Group. The effect of in- tensive treatment of diabetes on the development and the progression of long- term complications in insulin-dependent diabetes mellitus. N Eng. J Med. 1993; 329:977–78.
- Berger M. towards more physiological insulin therapy in the 1990s: a com- ment. Diabetes Res Clin Pract. 1989; 6:S25–S31.
- Mastick CC, Brady MJ, Printen JA, Ribon V, Saltiel AR. Spatial determinants of specificity in insulin action. Mol Cell Biochem. 1998; 182:65–71.
- Saltiel AR. Diverse signalling pathways in the cellular actions of insulin. Am J Physiol. 1996; 270:E375–E85.
- Azpiazu I, Saltiel AR, DePaoli-Roach AA, Lawrence JC. Regulation of both glycogen synthase and PHAS-I by insulin in rat skeletal muscle involves mitogen-activated protein kinase-independent and rapamycin-sensitive path- ways. J Biol Chem. 1996; 271:5033–39
- Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, Trub
- T. Correlations of receptor binding and mi togenic potencies of insulin analogs designed for clinical use. Diabetes. 2000; 49:999–05.
- Slieker LJ, Brooke GS, DiMarchi RD, Flora DB, Green LK, Hoffmann JA, Long HB, Fan L, Shields JE, Sundell KL, Surface PL, Chance RE. Modifica- tions in the B10 and B26–30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Dia- betologia. 1997; 40(Suppl 2):S54–S61.
- Baker EN, Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DM, Hubbard RE, Isaacs NW, Reynolds CD. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988; 319:369–456.
- Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28),Pro(B29)]- human insulin: a rapidly absorbed analogue of human insulin. Diabetes. 1994; 43:396–402.
- Torlone E, Fanelli C, Rambotti AM, Kassi G, Modarelli F, Di Vincenzo A, Epifano L, Ciofetta M, Pampanelli S, Brunetti P. Pharmacokinetics, pharma- codynamics and glucose counterregulation following subcutaneous injection of the monomeric insulin analogue [Lys(B28),Pro(B29)] in IDDM. Diabeto- logia. 1994; 37:713–20.
- Slieker LJ, Sundell K. Modifications in the 28–29 position of the insulin B-chain alter binding to the IGF-I receptor with minimal effect on insulin receptor binding. Diabetes. 1991; 40(Suppl 1):168A.
- Slieker LJ, Brooke GS, Chance RE. Insulin and IGF-I analogues: novel approaches to improved insulin pharmacokinetics. In: Le- Roith D, Raizada MK, eds. Current directions in insulin-like growth factor research. New York Plenum Press. 343:25–32.
- Pfutzner A, Kustner E, Forst T, Schulze-Schleppinghoff B, Trautmann ME, Haslbeck M, Schatz H, Beyer J. Intensive insulin therapy with insulin lispro in patients with type 1 diabetes reduces the frequency of hypoglycemic episodes. Exp Clin Endocrinol Diabetes. 1996; 104:25–30.
- Anderson Jr JH, Brunelle RL, Koivisto VA, Pfutzner A, Trautmann ME, Vig- nati L, DiMarchi R. Reduction of postprandial hyperglycaemia and frequency of hypoglycemia in IDDM patients on insulin-analogue treatment. Multicen- tre Insulin Lispro Study Group. Diabetes. 1997; 46:265–70.
- Brunelle BL, Llewelyn J, Anderson Jr JH, Gale EA, Koivisto VA. Meta-analy- sis of the effect of insulin lispro on severe hypoglycaemia in patients with type 1 diabetes. Diabetes. 1998; Care 21:1726–31.
- Karsidag K, Satman I, Dinccag N, Altunas Y, Karadeniz S, Yilmaz MT. Com- parison of metabolic control in IDDM with two different intensive regimens of [Lys(B28), Pro(B29)] human insulin (lispro) plus NPH insulin. Diabeto- logia. 1996; 39(Suppl 1):A222.
- Ciofetta M, Lalli C, Del Sindaco P, Torlone E, Pampanelli S, Mauro L, Chiara DL, Brunetti P, Bolli GB. Contribution of postprandial versus interprandial blood glucose to HbA1c in type 1 diabetes on physiologic intensive therapy with lispro insulin at mealtime. Diabetes Care. 1999; 22: 795–800.
- Del Sindaco P, Ciofetta M, Lalli C, Perriello G, Pampanelli S, Torlone E, Brunet- ti P, Bolli GB. Use of the short-acting insulin analogue lispro in intensive treat- ment of type 1 diabetes mellitus: importance of appropriate replacement of basal insulin and timeinterval injection-meal. Diabet Med. 1998; 15:592–600.
- Lalli C, Ciofetta M, Del Sindaco P, Torlone E, Pampanelli S, Compagnucci P, Cartechini MG, Bartocci L, Brunetti P, Bolli GB. Long-term intensive treatment of type 1 diabetes with the short-acting insulin analogue lispro in variable combination with NPH insulin at mealtime. Diabetes Care. 1999; 22:468–77.
- Jovanovic L, Ilic S, Pettitt DJ, Hugo K, Gutierrez M, Bowsher RR, Bastyr EJ. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care.1999; 22:1422–27.
- Calle-Pascual AL, Bagazgoitia J, Calle JR, Charro A, Maranes JP. Use of insulin lispro in pregnancy. Diabetes Nutr Metab. 2000; 13:173–77.
- Garg SK, Anderson JH, Perry SV, Mackenzie T, Keith P, Jennings MK, Hansen MM, Chase HP, Long-term efficacy of Humalog in subjects with Type 1 diabe- tes mellitus. Diabet Med. 1999; 16:384–87.
- Anderson J, Symanowski S, Brunelle R. Safety of [Lys(B28),Pro(B29) human insulin] analogue in long-term clinical trials. Diabetes. 1994; 43 (Suppl 1):61A.
- Fineberg NS, Fineberg SE, Anderson JH, Birkett MA, Gibson RG, Hufferd S. Immunologic effects of insulin lispro [Lys(B28),Pro(B29) human insulin] in IDDM and NIDDM patients previously treated with insulin. Diabetes. 1996; 45:1750–54.
- Roach P, Varshavsky JA, Gantner K, Anderson JH. Insulin antibody forma- tion during treatment with human insulin or insulin lispro does not affect insulin dose requirements. Diabetes. 1996; 45(Suppl 2):261A.
- Henrichs HR, Unger H, Trautmann ME, Pfutzner A. Severe insulin resistance treated with insulin lispro. Lancet. 1996; 348:1248.
- Lahtela JT, Knip M, Paul R, Antonen J, Salmi J. Severe antibody-mediated human insulin resistance: successful treatment with the insulin analogue lis- pro: a case report. Diabetes Care. 1997; 20:71–73.
- Drejer K, Kruse V, Larsen DU, Hougaard P, Bjorn S. Gameltoft S. Receptor binding and tyrosine kinase activation by insulin analogues with extreme af- finities studied in human hepatoma Hep G2 cells. Diabetes, 1991; 40:1488-95.
- Bolli GB, Di Marchi RD, Park GD, Pramming S Koivisto VA. Insulin analogues and their potentials in the management of Diabetes Mellitus. Diabetologia 1999; 42:1151-67.
- Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T, Time- action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000; 23:644–49A.
- Seipke GK, Geisen HP, Neubauer C, Pittius R, Rosskamp R, Schwabe D. New insulin preparations with prolonged action profiles: A21 modified arginine insulins. Diabetologia. 1992; 35(Suppl 1):A4.
- Berti L, Kellerer M, Bossenmaier B, Seffer E, Seipke G, Haring HU. The long- acting human insulin analogue HOE 901: characteristics of insulin signal- ling in comparison to Asp(B10) and regular insulin. Horm Metab Res. 1998; 30:123–29.
- Bahr M, Kolter T, Seipke G, Eckel J, Growth promoting and metabolic activity of the human insulin analogue[ GlyA21,ArgB31,ArgB32]insulin (HOE 901) in muscle cells. Eur J Pharmacol. 1997; 320:259–65.
- Dreyer M, Pein M, Schmidt B, Heidtmann B, Schlunzen M, Rosskamp R. Com- parison of the pharmacokinetics/dynamics of GLY(A21)-ARG(B31,B32)-hu- man-insulin (HOE71GT) with NPH-insulin following subcutaneous injection by using euglycemic clamp technique. Diabetologia. 1994; 37(Suppl 1):A78.
- Owens D, Luzio S, Beck P, Coates P, Tinbergen J, Kurzhals R. The absorption of the insulin analogue HOE 901 from different sites in healthy subjects. Dia- betes. 1997; 46(Suppl 1):329A.
- Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R. Pharmacokinet- ics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care. 2000; 23:813–19A.
- Talaulicar M, Willms B, Rosskamp R, HOE 901, the newer Insulin analog and Substitution des basalen Insulin-bedarfs bei Type I Diabetes. Diabetes and Stoffwechsel. 1996; 5:3–6
- Rosenstock J, Park G, Zimmermann J. Efficacy and safety of HOE 901 in patients with type 1 DM: a four-week randomized, NPH insulin-controlled trial. Diabetes. 1998; 47(Suppl 1):A92.
- Pieber T, Eugene-Jolchine I, DeRobert E. Efficacy and safety of HOE 901 in patients with type 1 diabetes: a four-week randomized, NPH insulin-control- led trial. Diabetes. 1998; 47(Suppl 1):A62.
- Raskin P, Park G, Zimmerman J. The effect of HOE 901 on glycemic control in type 2 diabetes. Diabetes. 1998; 47(Suppl 1):A103.
- Matthews DR, Pfeiffer C. new long-acting insulin (HOE 901) demonstrates less nocturnal hypoglycemia when compared with protamine insulin in a clini- cal trial. Diabetologia. 1998; 41(Suppl 1):A245.
- Rosenstock J, Schwartz S, Clark C, Edwards M, Donley D. Efficacy and safety of HOE 901 (Insulin Glargine) in subjects with type 2 DM: a 28-week randomized, NPH insulin-controlled trial. Diabetes. 1999;48 (Suppl 1): A100.
- Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycaemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000; 23:639–43A.
- Pieber TR, Eugene-Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. The European Study Group of HOE 901 in type 1 diabetes. Diabetes Care. 2000; 23:157–62.
- Rosenstock J, Park G, Zimmerman J. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insu- lin regimens. U.S. Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care. 2000; 23:1137–42.
- Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabe- tes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–36.
- Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspen- sion of neutral protamine Hagendorn (NPH) insulin in pens. Lancet. 1999; 6:1604–07.
- Brange J, Ribel U, Hansen JF, Dodson G, Hansen MT, Havelund S, Melberg SG, Norris F, Norris K, Snel L. Monomeric insulins obtained by protein engi- neering and their medical implications. Nature. 1988; 333:679–82.
- Drejer K. The bioactivity of insulin analogues from in vitro receptor binding to in vivo glucose uptake. Diabetes Metab Rev. 1992; 8:259–86.
- Kang S, Creagh FM, Ara J, Owens DR Peters JR. Insulin analogues and human insulin: near-equivalent in vivo biological activity in healthy males in spite of widely different in vitro potencies. Diabetes. 1991; 40 (Suppl 1): 243A.
- Dall V. Preclinical safety pharmacology studies on the rapid-acting insulin analogue insulin aspart. Arzneimittelforschung. 1999; 49:463–70.
- Frier BM, Ewing FM, Lindholm A, Hylleberg B, Kanc K. Symptomatic and counterregulatory hormonal responses to acute hypoglycaemia induced by in- sulin aspart and soluble human insulin in Type 1 diabetes. Diabetes Metab Res Rev. 2000; 16:262–68.
- Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998; 21:1910–14.
- Lindholm A, McEwen J, Riis AP. Improved postprandial glycemic control with insulin aspart: a randomized double-blind cross-over trial in type 1 dia- betes. Diabetes Care. 1999; 22:801–05.
- Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, Henry RR. Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999; 22:1501–06.
- Raskin P, Guthrie RA, Leiter L, Riis A, Jovanovic L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care. 2000; 23:583–88.
- Home PD, Lindholm A, Hylleberg B, Round P. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. UK Insulin Aspart Study Group. Diabetes Care. 1998; 21:1904–09.
- Brunner GA, Hirschberger S, Sendlhofer G, Wutte A, Ellmerer M, Balent B, Schaupp L, Krejs GJ, Pieber TR. Post-prandial administration of the insulin analogue insulin aspart in patients with Type 1 diabetes mellitus. Diabet Med. 2000; 17:371–75.
- Rosenfalck AM, Thorsby P, Kjems L, Birkeland K, Dejgaard A, Hanssen KF, Madsbad S. Improved postprandial glycaemic control with insulin aspart in type 2 diabetic patients treated with insulin. Acta Diabetol. 2000; 37:41–46.
- Mortensen HB, Lindholm A, Olsen BS, Hylleberg B. Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur J Pediatr. 2000; 159:483–88
- Weyer C, Heise T, Heinemann L. Insulin aspart in a 30/70 premixed formula- tion: pharmacodynamic properties of a rapid-acting insulin analog in stable mixture. Diabetes Care. 1997; 20:1612–14.
- Jacobsen LV, Sogaard B, Riis A. Pharmacokinetics and pharmacodynamics of a premixed formulation of soluble and protamine-retarded insulin aspart. Eur J Clin Pharmacol. 2000; 56:399–03.
- Pieber TR, Eugene-Jolchine I, Derobert E. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes: the European Study Group of HOE 901 in type 1 diabetes. Diabetes Care 2000; 23: 157–62.
- Raskin P, Klaff L, Bergenstal R, Halle JP, Donley D, Mecca T. A 16-week compar- ison of the novel insulin analog insulin glargine. Diabetes Care. 2000; 23:1666–71.
- Rosenstock J, Park G, Zimmerman J. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens:
- U.S. Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Diabetes Care 2000; 23: 1137–42.
- Lindholm E. New insulins in the treatment of diabetes mellitus. Best Pract Res Clin Gastroenterol 2002; 16:475–92.
- Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet. 2008; 47(1):7–20.
- Becker RH. Insulin glulisine complementing basal insulins: a review of structure and activity. Diabetes Technol Ther. 2007; 9(1):109–21.