Retrospective analysis of antibiotic resistance pattern to urinary pathogens in a Tertiary Care Hospital in South India
- *Corresponding Author:
- Dr. Saligrama Chikkannasetty Somashekara,
Department of Pharmacology, Malabar Medical College and Research Centre, Modakkallur, Kozhikode -673 315, Kerala, India.
E-mail: drsomashekara@gmail.com
Abstract
Context: The distribution of uropathogens and their susceptibility pattern to antibiotics vary regionally and even in the same region, they change over time. Therefore, the knowledge on the frequency of the causative microorganisms and their susceptibility to various antibiotics are necessary for a better therapeutic outcome. Aim: The aim was to study the frequency and distribution of uropathogens and their resistance pattern to antibiotics in a tertiary care hospital. Settings and Design: Retrospective study for a period of 1 year from January 2011 to December 2011 in a tertiary care hospital. Materials and Methods: The culture and sensitivity data of the uropathogens from suspected cases of UTI were collected from the records of Microbiology Department for study period. Midstream urine samples were processed for microscopy and culture, and the organisms were identified by standard methods. Antibiotic susceptibility was carried out by Kirby‑Bauer disk diffusion method according to Clinical and Laboratory Standards Institute guidelines. Descriptive statistics were used to analyze the data. Results: Of 896 urine samples, 348 (38.84%) samples were positive for urine culture. Escherichia coli (52.59%) was the most common organism followed by Klebsiella. E. coli was least resistant to imipenem (8%) and amikacin (16%) and was highly resistant to co‑trimoxazole (69%) and ampicillin (86%). Klebsiella species were least resistant to amikacin (26%) and were highly resistant to ampicillin (92%). The overall resistance pattern of antibiotics to uropathogens was the highest to nalidixic acid (79%) followed by co‑trimoxazole (75%) and ampicillin (72%). Good susceptibility was seen with imipenem and cephalosporins. Conclusion: E. coli is still the most common uropathogen. Nalidixic acid, ampicillin, co‑trimoxazole, and first‑generation fluoroquinolones have limited value for the treatment of UTI. Sensitivity to imipenem and amikacin are still retained and may be prescribed for complicated UTI. Routine monitoring of drug resistance pattern will help to identify the resistance trends regionally. This will help in the empirical treatment of UTIs to the clinicians.
Keywords
Antibiotics, resistance, uropathogens
Introduction
Urinary tract infections (UTI) are the second most common infections after the infections of the respiratory tract.[1] UTI is the most common in patients with diabetes and in those with structural and neurological abnormalities which interfere with urinary outflow. Nosocomial UTI is also common following catheterization and cystoscopy. The manifestations of UTI may vary from mild asymptomatic cystitis to pyelonephritis and septicemia.[2] Gram‑negative organisms are the most common pathogens involved in UTI, but almost all known pathogens have been incriminated as possible causative agents for UTI.[3,4]
Treatment of UTI constitutes a great portion of prescription of antibiotics. Urinary pathogens have shown a changed pattern of susceptibility to antibiotics, resulting in an increase in resistance to commonly used antibiotics.[5]
The distribution of uropathogens and their susceptibility pattern to antibiotics vary regionally.[6,7] Therefore, the knowledge on the frequency of the causative microorganisms and their susceptibility to various antibiotics are necessary. Hence, the current retrospective analysis of the uropathogens and their susceptibility pattern during 1 year in patients with UTI in a tertiary care hospital has been undertaken.
Materials and Methods
Retrospective study on the resistance pattern of uropathogens for 1 year (January 2011 to December 2011) was done from suspected cases of UTI. The culture and sensitivity reports were collected from the records of Microbiology Department for study period. Approval from the Institutional Ethics Committee was obtained prior to the study. A total of 896 urine samples was analyzed for culture and sensitivity. Midstream urine samples were collected in sterile containers. The samples were cultured on blood agar and MacConkey media with a standard loop and were incubated at 37°C overnight. A growth of ≥ 105 colony forming units/mL was considered as significant bacteriuria.
The isolates were identified by Gram‑staining and conventional biochemical methods.[8] Antimicrobial susceptibility was done by Kirby–Bauer disc diffusion method on Mueller– Hinton agar and the interpretations were carried out according to the Clinical and Laboratory Standards Institute guidelines.[9] Antibiotics against which sensitivity was tested included ampicillin, amoxyclav, amikacin, cefotaxime, ceftazidime, imipenem, co‑trimoxazole, ciprofloxacin, levofloxacin, norfloxacin, nalidixic acid, and ofloxacin. All data were tabulated and analyzed. Descriptive statistics were used for analysis, and the results were expressed as frequency and percentage. Microsoft Excel 2007 software was used to analyze the data.
Results
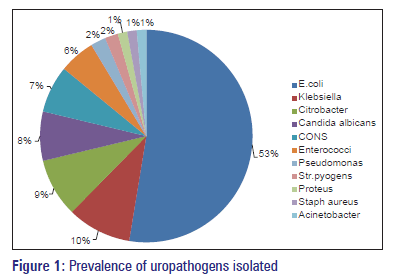
Of 896 urine samples analyzed, 548 (61.16%) were from females and 348 (38.84%) were from males. Of 896 urine samples, 348 (38.84%) samples were positive for urine culture. Escherichia coli (52.59%) was the most common organism followed by Klebsiella, Citrobacter and Staphylococcus aureus. Proteus and Acinetobacter showed the least infectivity pattern accounting only for 1.44% of the organisms isolated. Candida albicans growth was seen in 7.47% of the samples. The pattern of bacterial agents isolated is as shown in Figure 1.
Antibiotic susceptibility test
Escherichia coli was least resistant to imipenem (8%) and amikacin (16%), moderate to ceftazidime (36%) and showed high resistance pattern to co‑trimoxazole (69%), fluoroquinolones, and ampicillin (86%). Klebsiella species were least resistant to amikacin (26%), moderate to cephalosporins and fluoroquinolones, and highly resistant to ampicillin (92%). Citrobacter species showed good susceptibility only to Imipenem and moderate susceptibility to the remaining antibiotics. The antibiotic susceptibility patterns of the remaining organisms are shown in Table 1.
| Organisms isolated | Antibiotic resistance pattern (% resistance) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | AC | AK | CE | CA | I | CI | LE | OF | CO | NA | NX | |
| E. coli | 85.6 | 70 | 16 | 36.3 | 58.2 | 8 | 72 | 76.5 | 64.2 | 68.8 | 62 | 78.6 |
| Klebsiella | 92.4 | 68 | 26 | 38.4 | 42.2 | 12.2 | 42.2 | 31 | 35.2 | 52.2 | 48.2 | 38 |
| Citrobacter | 56 | 52 | 23.2 | 42.8 | 36.3 | 8.2 | 52.1 | 43.6 | 54.3 | 86.5 | 73.2 | 76.4 |
| CONS | 86 | 82 | ND | 32 | 24 | 6 | 64 | 46 | 52 | 90 | 90 | 56 |
| Enterococci | 36.4 | 18.2 | ND | 12 | 14.7 | 8.2 | 67 | 56.2 | 54 | 24.2 | 74.2 | 68.2 |
| Pseudomonas | 98 | 96.2 | 36 | 18.2 | 16 | 68 | 84.4 | 82.6 | 84.2 | 100 | 96.2 | 92 |
| S. pyogens | 46.3 | 34.8 | ND | 53.2 | 41.8 | 4 | 67.2 | 53.5 | 64.2 | 76 | 98.5 | 56 |
| Proteus | 72 | 69.5 | 28 | 37.2 | 26.8 | 6 | 18.6 | 32 | 64 | 98 | 88 | 26.4 |
| S. aureus | 78 | 72 | ND | 32.5 | 36.2 | 14 | 46 | 38.4 | 68.2 | 64 | 84 | 62.6 |
| Acinetobacter | ND | ND | 26.2 | 62.4 | 42 | 13 | 64.3 | 54 | 62 | 92.4 | 74 | 72.2 |
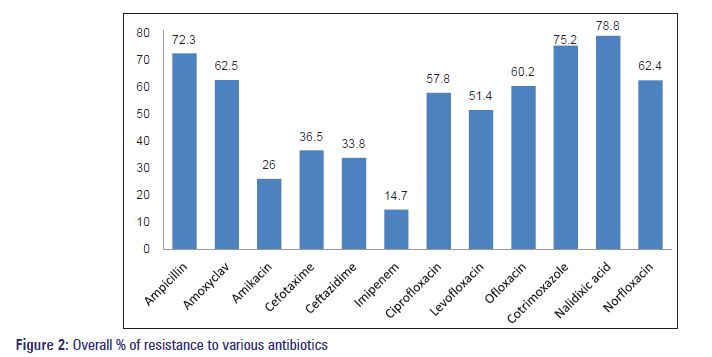
| Overall resistance (%) | 72.3 | 62.5 | 26 | 36.5 | 33.8 | 14.7 | 57.8 | 51.4 | 60.2 | 75.2 | 78.8 | 62.4 |
A: Ampicillin, AC: Amoxyclav, AK: Amikacin, CE: Cefotaxime, CA: Ceftazidime, I: Imipenem, CI: Ciprofloxacin, LE: Levofloxacin, OF: Ofloxacin,
CO: Co-trimoxazole, NA: Nalidixic acid, NX: Norfloxacin, ND: Not done, E. coli: Escherichia coli, S. pyogens: Streptococcus pyogenes, S. aureus: Staphylococcus aureus; CONS: Coagulase negative staphylococcus aureus
Table 1: Antibiotic resistance pattern of uropathogens to various antibiotics.
The overall resistance pattern of antibiotics to uropathogens was the highest to nalidixic acid (79%) followed by Co‑trimoxazole (75%) and Ampicillin (72%). Moderate susceptibility was seen with fluoroquinolones, and good susceptibility was seen with Imipenem (15%) and Cephalosporins [Figure 2].
Discussion
Gram‑negative organisms are the most common organisms causing UTIs, and they collectively account for more than 75% of cases. The spectrum of uropathogens isolated from urine samples in this study is very similar to the studies done in different regions of India and also that reported in the literature.[10]
Escherichia coli is the most common uropthogen accounting for 53% of cases. The incidence of E. coli as a causative pathogen in India varies from 48% to 65% as reported by various studies done earlier.[6,11] Klebsiella is the second most common uropathogen accounting for 10% of cases. The incidence of Klebsiella as uropathogen varies from 8% to 26%.[6,11] Other organisms collectively account for 15–20% of cases. Candida species growth is about 7%. This may be due to increased prevalence of HIV/AIDS, diabetes, and with the indiscriminate use of broad spectrum antibiotics.
In our study, 86% of E. coli isolates were found to be resistant to Ampicillin. Such high level of resistance to ampicillin was documented from various studies from different parts of India. A study done in the northern part of India reported 76% of Ampicillin resistance.[12] Other studies done in South India, reported a high prevalence of Ampicillin resistance. A study from Karnataka had reported 80.6% resistance, and another study from Tamil Nadu reported 96.2% of Ampicillin resistance.[13,14]
The resistance of E. coli to co‑trimoxazole is 68.8% in our study. Kasi Murugan et al.[14] from Tamil Nadu and Manjunath et al.[13] from Karnataka reported the resistance pattern to be 70.4% and 47.9%, respectively. The decreased resistance pattern to co‑trimoxazole in Karnataka may be due to the decreased use of co‑trimoxazole as empirical therapy for UTIs.
There is increased resistance pattern of E. coli to Fluoroquinolones. The similar observations were reported from the studies from other parts of India. This may be due to the widespread use of fluoroquinolones as first‑line empirical therapy for UTIs.
Considerable sensitivity is still retained to imipenem and amikacin due to less use of these injectable antibiotics.
Moderate sensitivity was found with cephalosporins and newer fluoroquinolones.
In our study, the highest resistance by Klebsiella was noted against ampicillin, followed by amoxyclav, co‑trimoxazole and fluoroquinolones. Moderate resistance was noted against cephalosporins, levofloxacin and was least resistant to amikacin and imipenem. Similar resistance pattern was noted by various workers from different parts of India.[11‑14]
The resistance pattern to other organisms in our study is similar to the other studies from the different parts of India.[11‑14]
The overall resistance pattern of antibiotics to uropathogens from other studies done in different parts of India is compared in Table 2. From all the studies, it is evident that the uropathogens are least resistant to amikacin and imipenem. This may be due to the less common use of these injectable antibiotics. Moderate resistance is seen with cephalosporins and third‑generation fluoroquinolones. More resistant pattern is seen with ampicillin, amoxiclav, co‑trimoxazole and the first‑ and second‑generation fluoroquinolones. This may be due to the wide use of these antibiotics as empirical therapy for the treatment of UTIs.
| Various studies | Overall antimicrobial resistance pattern (% resistance) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| across India | A | AC | AK | CE | CA | I | CI | LE | OF | CO | NA | NX |
| Present study | 72.3 | 62.5 | 26 | 36.5 | 33.8 | 14.7 | 57.8 | 51.4 | 60.2 | 75.2 | 78.8 | 62.4 |
| Krishna et al.[15] | 82 | 72 | 15 | - | 60 | - | 23 | - | - | - | - | - |
| Murugan et al.[14] | 67.4 | - | 33.4 | 43.8 | - | - | 43.5 | 45 | 50.2 | - | 82.6 | 47 |
| Mandal et al.[17] | 71.5 | - | 39.1 | 61.5 | 50.1 | 17.8 | 57.3 | - | - | - | - | - |
| Shalini et al.[16] | 77.4 | 64.3 | 12.6 | - | 26.6 | - | 26.6 | 26.6 | - | 81.8 | - | 27.3 |
| Manjunath et al.[13] | 80.4 | - | 6.9 | 46.5 | 43.2 | 7.5 | 73 | - | 7.7 | 47.9 | - | 53.3 |
A: Ampicillin, AC: Amoxyclav, AK: Amikacin, CE: Cefotaxime, CA: Ceftazidime, I: Imipenem, CI: Ciprofloxacin, LE: Levofloxacin, OF: Ofloxacin,
CO: Co-trimoxazole, NA: Nalidixic acid, NX: Norfloxacin.
Table 2: Comparative antimicrobial resistance pattern of uropathogen from different parts of India.
The major limitation of the study is that it did not distinguish the distribution of organisms in the community acquired UTI and nosocomial UTI. As a consequence, the prevalence of microorganisms and their resistance pattern in both types of UTI could not be ascertained.
Conclusion
The retrospective study furnished the details about common uropathogens and their drug resistance pattern. From the study, it is clear that, E. coli is still the most common uropathogen. Antibiotics such as nalidixic acid, ampicillin, co‑trimoxazole, and first‑generation fluoroquinolones have limited value for the treatment of UTI. Sensitivity to imipenem and amikacin are still retained and may be prescribed for complicated UTI.
Hence, routine monitoring of susceptibility patterns is necessary. This will help in the empirical treatment of UTI to the clinicians and also for the preparation of antibiotic policy of the individual institute. This will avoid the indiscriminate use of antibiotics and prevent the further development of antimicrobial resistance.
Acknowledgements
The authors would like to thank the staffs and postgraduate students of the Microbiology Department for their kind co‑operation during the study.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 773-805.
- Naveen R, Mathai E. Some virulence characteristics of uropathogenic Escherichia coli in different patient groups. Indian J Med Res 2005;122:143-7.
- Wilkie ME, Almond MK, Marsh FP. Diagnosis and management of urinary tract infection in adults. BMJ 1992;305:1137-41.
- Bajaj JK, Karyakarte RP, Kulkarni JD, Deshmukh AB. Changing aetiology of urinary tract infections and emergence of drug resistance as a major problem. J Commun Dis 1999;31:181-4.
- Magalit SL, Gler MT, Tupasi TE. Increasing antimicrobial resistance patterns of community and nosocomial uropathogens in Makati Medical Center. Philipp J Microbiol Assoc 2001;51:94-100.
- Farrell DJ, Morrissey I, De Rubeis D, Robbins M, Felmingham D.A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect 2003;46:94-100.
- Mathai D, Jones RN, Pfaller MA, SENTRY Participant Group North America. Epidemiology and frequency of resistance among pathogens causing urinary tract infections in 1,510 hospitalized patients: A report from the SENTRY Antimicrobial Surveillance Program (North America). Diagn Microbiol Infect Dis 2001;40:129-36.
- Collee JG, Miles RS, Watt B. Test for identification of bacteria. In:Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York:Churchill Livingstone; 1996. p. 131-49.
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing; 16th Informational Supplement. M100-S16. Wayne, PA: Clinical and Laboratory Standards Institute; 2006.
- Nicolle LE. Epidemiology of urinary tract infections. Infect Med 2001;18:153-62.
- Hasan AS, Nair D, Kaur J, Baweja G, Deb M, Aggarwal P. Resistance patterns of urinary isolates in a tertiary Indian hospital. J Ayub Med Coll Abbottabad 2007;19:39-41.
- Gupta N, Kundra S, Sharma A, Gautam V, Arora DR. Antimicrobial susceptibility of uropathogens in India. J Infect Dis Antimicrob Agents 2007;24:13-8.
- Manjunath GN, Prakash R, Annam V, Shetty K. Changing trends in the spectrum of antimicrobial drug resistance pattern of uropathogens isolated from hospitals and community patients with urinary tract infections in Tumkur and Bangalore. Int J Biol Med Res 2011;2:504-7.
- Murugan K, Savitha T, Vasanth S. Retrospective study of antibiotic resistance among uropathogens from rural teaching hospital, Tamilnadu, India. Asian Pac J Trop Dis 2012;2:375-80.
- Krishna S, Pushpalatha H, Srihari N, Nagabhushan S, Divya P. Increasing resistance patterns of pathogenic bacteria causing urinary tract infections at a tertiary care hospital. Int J Pharm Biomed Res 2013;4:105-7.
- Shalini, Joshi MC, Rashid MK, Joshi HS. Study of antibiotic sensitivity pattern in urinary tract infection at a tertiary hospital. Natl J Integr Res Med 2011;2:43-6.
- Mandal J, Acharya NS, Buddhapriya D, Parija SC. Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin resistant Escherichia coli. Indian J Med Res 2012;136:842-9.