Prevalence and Susceptibility Analysis of Carbapenem Resistant Gram Negative Pathogens in Tertiary Care Hospital, Mumbai
Citation: Sriram A, Tulara NK. Prevalence and Susceptibility Analysis of Carbapenem Resistant Gram Negative Pathogens in Tertiary Care Hospital, Mumbai. J Basic Clin Pharma 2018;9:pp-pp.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Background and objective: Antibiotic resistance has risen perilously in all parts of the world. The lack of appropriate therapeutics to encounter resistant pathogens has enhanced the urge for the development of either new antibiotics or adjuvant therapy with antibiotics. Thus, we aimed to study a comparative antibiogram pattern of 102 clinical isolates towards Elores (a novel antibiotic and adjutant entity of ceftriaxone, sulbactam and disodium edetate) and other antibiotics (colistin, piperacillin/tazobactam, tigecycline, cefoperazone+sulbactam and cefepime+tazobactam). Methods: The clinical samples collected from infective patients admitted to Tertiary care Hospital, Mumbai (India) between June 2016 to December 2016 was further subjected to bacterial identification. Antibiotic susceptibility testing was executed in accordance with the recommendations of Clinical Laboratory Standards Institute (CLSI) guidelines. All isolates included in this study were resistant to carbapenems. Results: Out of 4212 collected samples, carbapenem resistant isolates were recovered from 102 clinical samples in which urine samples contributed 51.96% followed by sputum and tracheal secretion which added 8.82% each while rest of the clinical specimen contributed 30.4%. Klebsiella pneumoniae (61.76%) was most predominant among all the resistant clinical isolates followed by Escherichia coli (18.63%), Acinetobacter baumannii (14.71%), Serratia marcescens (2.94%) and Enterobacter cloacae (1.96%). These 102 clinical isolates which were found resistant towards imipenem and meropenem were included in this study and further processed for antimicrobial susceptibility analysis with respect to other antibiotics. Data suggested, the antibiogram profile of Elores was extremely higher (100% susceptible) towards clinical pathogens over rest of the antibiotics such as piperacillin/tazobactam (0-6.7%), cefoperazone+sulbactam (0-20%), cefepime+tazobactam (0-33.3%) and tigecycline (34.7-100%) but it was found comparable to colistin (87.3-100%) except S. marcescens which shows inherent resistance to colistin. Conclusion: Susceptibility profile data revealed the equivalence of Elores (Antibiotic-adjuvant entity; AAE) with colistin and strong superiority over other antibiotics including β-lactam and β-lactamase inhibitors combination (BL-BLI) and protein synthesis inhibitors. Hence, Elores can be treated as a most efficient treatment option towards infections caused by carbapenem resistant pathogens.
Keywords
Antibiotic, clinical isolates, elores, prevalence, susceptibility, resistance
Introduction
The role of antibiotics is not limited to save patients from infection but also has role in surgery which includes chemotherapy treatment, organ transplants, cardiac surgery, rheumatoid arthritis, diabetes etc.[1-3] In fact antibiotics have helped to decrease the morbidity and mortality rates in developing countries thus extending the human life span.[4,5] But unfortunately in recent decades a medical threat i.e., resistance against antibiotics has been observed towards most of the antibiotics which has become the major issue in combating against clinical pathogens.[6] Overuse of antibiotics, inappropriate processing, extensive agricultural use and lack of regulatory barriers are the major causes for antibiotic resistance.[7] At present, antibiotic resistance has become a global threat that may pass from one species of bacteria to another through various genetic methods including horizontal gene transfer.[8]According to the studies E. coli has been reported with a huge rise in resistance against many antibiotic like carbapenems, third generation cephalosporin and fluoroquinolone and up to 66.6%, 83% and 85% respectively.[9,10] In North-east India, E. coli have also been found resistant to doripenem, a carbapenem antibiotic, which exhibited 78.57% resistance.[11] Likewise, K. pneumoniae clinical isolates have also displayed resistance at a very high scale i.e., 80%, 73% and 52% towards third generation cephalosporin, fluoroquinolone and carbapenems respectively.[12] Extended-spectrum beta-lactamases (ESBL) carrying K. pneumoniae clinical isolates have also shown resistance towards cefoperazone and cefepime up to 70-100% compared to the ESBL non-producer (0-25%).[13,14] A recent study from Mumbai, India also depicted the similar antibiotic resistance in A. baumannii which depicted enhanced resistance in A. baumannii due to the routine use of antibiotics including tigecycline, piperacillin and colistin.[15] Acienetobacter species isolated from blood, pus, urine and sputum were also found to exhibit 58.34% resistance towards piperacillin/ tazobactam.[16]
Based on the present data of antibiotic resistance, there is a keen requirement of either new antibiotics or adjuvant therapy along with antibiotics which should have the potential to prevent this medical threat by treating infectious diseases and help mankind. Herein, we aimed to study the susceptibility pattern of Elores (a novel antibioticadjuvant entity of ceftriaxone, sulbactam and disodium edetate) in comparison to the different classes of antibiotics polymixins (colistin), BL-BLI (piperacillin/tazobactam, cefoperazone+sulbactam and cefepime+tazobactam) and protein synthesis inhibitors (tigecycline) against carbapenem resistant pathogens.
Material and Methods
Sample collection
Various clinical samples including urine, wound, endotracheal tube (ET) secretion, central line, tissue, cerebrospinal fluid (CSF), tracheal secretion, ICD fluid, ascitic fluid, bile, sputum, pus, high vaginal swab and pleural fluid were collected from patients at Tertiary care Hospital, Mumbai (India), during the period of June 2016 to December 2016. The collection and processing of the samples were done as per SOP.
Isolation and identification of microbes
All the clinical samples were collected aseptically from the infected body sites of the patients and inoculated on the various selective and non-selective culture media as per the standard microbiological techniques. Different selective culture media were used for the isolation of microorganisms such as sheep blood agar, sheep chocolate agar, MacConkey agar medium. Organisms were categorized based on colony morphology, gram staining and identification was done using Vitek 2 system (Biomerieux).
Antibiotic susceptibility testing
The clinical isolates which were resistant or showed intermediate susceptibility against imipenem and meropenem were included in this study and further antimicrobial susceptibility study was performed on theses isolates by Vitek-2 system and Kirby–Bauer disk diffusion method as recommended by the Clinical Laboratory Standards Institute (CLSI) guidelines.[17] In brief, inoculum of 0.5 McFarland standards turbidity was prepared in saline from isolated colony of pathogens selected from 18-24 hour agar plates. A sterile cotton swab was dipped into the inoculum and streaked three times on the dried surface of a Mueller-Hinton agar (MHA) plate. After 5 minutes, antibiotic discs were applied and pressed down to check absolute contact with agar surface. The discs were apportioned in a minimum distance of 24 mm from the centre. The plates were then incubated for 16-18 hrs aerobically at 37°C. The discs of Elores (45 μg) and cefepime+tazobactam (40 μg) were obtained from HiMedia, India and used in the study. Colistin, piperacillin-tazobactam, cefoperazone+sulbactam and tigecycline were tested by MIC using Vitek 2 system.
Results and Discussion
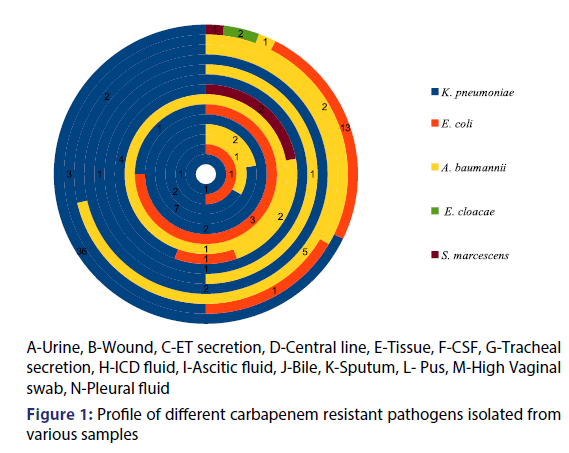
Out of the collected clinical isolates recovered from the various clinical specimens, 102 carbapenem resistant gram negative bacteria were used in this study in which the highest occurrence of resistant pathogens was found in urine samples (51.96%) followed by tracheal secretion and sputum (8.82% each), ET secretion (5.88%), ascitic fluid (3.92%), pus (2.94%) whereas clinical samples of central line, tissue and high vaginal swab exhibited less prevalence (1.96% each) and samples collected from CSF, ICD fluid and pleural fluid contributed the least prevalence i.e., 0.98% each [Table 1].
| Sr. No. | Name of clinical samples | Number of Pathogens (%) |
|---|---|---|
| 1 | Urine | 53 (51.96) |
| 2 | Wound | 6 (5.88) |
| 3 | Endotracheal tube (ET) Secretion | 7 (6.86) |
| 4 | Central Line | 2 (1.96) |
| 5 | Tissue | 2 (1.96) |
| 6 | Cerebrospinal fluid (CSF) | 1 (0.98) |
| 7 | Tracheal secretion | 9 (8.82) |
| 8 | ICD fluid | 1 (0.98) |
| 9 | Ascetic fluid | 4 (3.92) |
| 10 | Bile | 2 (1.96) |
| 11 | Sputum | 9 (8.82) |
| 12 | Pus | 3 (2.94) |
| 13 | High vaginal swab | 2 (1.96) |
| 14 | Pleural fluid | 1 (0.98) |
| Total | 102 |
Table 1: A profile of clinical samples used as a source of the carbapenem resistant pathogens
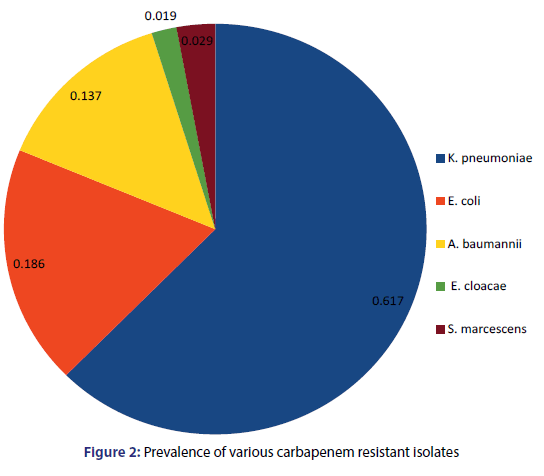
On the basis of morphological and biochemical screening five different pathogens were isolated included K. pneumoniae, E. coli, A. baumannii, E. cloacae and S. marcescens. Figure 1 depicts the detailed side view of the various carbapenem resistant clinical pathogens isolated from various clinical samples. Among all the five pathogens, K. pneumoniae (61.76%) was found to be most prevalent in all the clinical samples followed by E. coli (18.63%) and A. baumannii (14.71%) while E. cloacae (1.96%) and S. marcescens (2.94%) were least prevalent [Figure 2]. Akter et al. Revealed through their study that in recent few years K. pneumoniae has become a major and frequent opportunistic pathogen in hospital-associated infections.[18] Chaudhary et al. Have shown extreme prevalence of K. pneumoniae (63.1%) which favours our present study.[19] Our data demonstrated 18.63% prevalence of E. coli which is in accordance with Chaudhary and Payasi who determined 18.6% presence of E. coli among all clinical specimens.[20] Our present data showed the 13.7% prevalence of A. baumannii which is supported by Darvishi who demonstrated 13.3% occurrence of A. baumannii in clinical infectious samples.[21] Kumar et al. in their study observed 0.35% E. cloacae and 2.24% S. marcescens prevalence in clinical samples which confirms our present report.[22]
Table 2 represented the prevalence of different clinical isolates in different samples. K. pneumoniae has shown maximum occurrence in sputum (77.8%) and urine (67.9%). It has been considered as the major source for hospital acquired infections especially in nosocomial diseases. Romanus and Egwu reported that among all the clinical samples studied, sputum was containing highest percentage of Klebsiella spp (47.1%).[23] The same data was supported by Shilpa et al. who also determined the high ratio of K. pneumonia isolated from sputum (23%).[24] Barakzahi et al. Observed the high frequency of K. pneumoniae in urine (50%) which is in accordance with our findings.[25]
| Samples | Clinical isolates | |||||
|---|---|---|---|---|---|---|
| No. of isolates | K. pneumoniae (%) | E. coli (%) | A. baumannii (%) | E. cloacae (%) | S. marcescens (%) | |
| Urine | 53 | 36 (67.9) | 13 (24.5) | 1 (1.9) | 2 (3.7) | 1 (1.9) |
| Wound | 6 | 3 (50) | 1 (16.7) | 2 (33.3) | 0 | 0 |
| ET Secretion | 7 | 2 (28.6) | 0 | 5 (71.4) | 0 | 0 |
| Central Line | 2 | 2 (100) | 0 | 0 | 0 | 0 |
| Tissue | 2 | 1 (50) | 0 | 1 (50) | 0 | 0 |
| CSF | 1 | 1 (100) | 0 | 0 | 0 | 0 |
| Tracheal secretion | 9 | 4 (44.4) | 1 (11.1) | 2 (22.2) | 0 | 2 (22.2) |
| ICD fluid | 1 | 0 | 0 | 1 (100) | 0 | 0 |
| Ascitic fluid | 4 | 1 (25) | 3 (75) | 0 | 0 | 0 |
| Bile | 2 | 2 (100) | 0 | 0 | 0 | 0 |
| Sputum | 9 | 7 (77.8) | 0 | 2 (22.2) | 0 | 0 |
| Pus | 3 | 2 (66.7) | 0 | 1 (33.3) | 0 | 0 |
| High vaginal swab | 2 | 1 (50) | 1 (50) | 0 | 0 | 0 |
| Pleural fluid | 1 | 1 (100) | 0 | 0 | 0 | 0 |
| Total | 102 | 63 | 19 | 15 | 2 | 3 |
Table 2: Prevalence of different carbapenem resistant isolates in different samples
Present data reported extreme prevalence of E. coli in urine (24.5%) and wound (16.7%). The prevalence of ESBL producing E. coli has augmented globally and have become the leading root of treatment failure in ICUs. Mehta et al. Reporting high prevalence of E. coli in urine (40%).[26] Kibret et al. Have shown 18.7% prevalence of E. coli in wound samples.[27] On the other hand A. baumannii was the most prevalent in ET secretion (71.4%) followed by tracheal secretion (22.2%), wound (22.2%) and sputum (22.2%). A. baumannii can be found in respiratory, urinary, gastrointestinal tract and wound samples and displays a particular penchant for the ICU patients (Towner). Jaggi et al. During their study, isolated maximum isolates of A. baumannii from respiratory secretions (57.4%).[28] Kaur et al. Also reported the supreme prevalence of A. baumannii in ET secretion and sputum.[29]
S. marcescens were observed in tracheal secretion (22.2%) while E. cloacae were found in urine (3.7%). Vetter et al. reported a nosocomial eruption of S. marcescens in respiratory samples predominantly from suspected patients in ICU which are acknowledged as a causative agent of intense nosocomial and surgical site infections[30] and Leski et al. Displayed the presence of E. cloacae in urine samples of the patients which coincides with our findings.[31]
Table 2 depicts the prime prevalence (50-100%) of K. pneumoniae in the clinical samples including urine, central line, CSF, bile, sputum, pus and pleural fluid where Elores (AAE) exhibited 100% susceptibility against K. pneumoniae as compared to the other antibiotics (tigecycline, piperacillin/tazobactam, cefoperazone+sulbactam and cefepime+tazobactam). Likewise Elores contributed similar output (100% sensitivity) in the case of A. baumannii which displayed high prevalence (50-100%) in ICD fluid, ET secretion and tissue samples. From the present study it is evident that Antibiotic adjuvant therapy is superior to other commonly used classes of antibiotics (protein synthesis inhibitors, BL-BLI) in carbapenem resistant strains [Table 3].
| Susceptibility (%) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic Adjuvant Entity | β-lactam and β-lactamase inhibitor combinations | Polymixins | Protein Synthesis Inhibitors | |||||||||||||||
| Clinical isolates | Elores | Cefepime+Tazobactam | Piperacillin+Tazobactam | Cefoperazone+Sulbactam | Colistin | Tigecycline | ||||||||||||
| S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | |
| K. pneumoniae (n=63) |
100 | - | 0 | 6.3 | 1.6 | 92.1 | 1.6 | - | 98.4 | 0 | - | 100 | 87.3 | - | 12.7 | 34.7 | 19.2 | 46.1 |
| E. coli (n=19) |
100 | - | 0 | 15.8 | - | 84.2 | 0 | - | 100 | 0 | - | 100 | 100 | - | 0 | 100 | - | 0 |
| A. baumannii (n=15) |
100 | - | 0 | 20 | 13.3 | 66.7 | 6.7 | - | 93.3 | 20 | 20 | 40 | 100 | - | 0 | 92.9 | 7.1 | 0 |
| E. cloacae (n=2) |
100 | - | 0 | 0 | - | 100 | 0 | - | 100 | 0 | - | 100 | 100 | - | 0 | - | - | - |
| S. marcescens (n=3) |
100 | - | 0 | 33.3 | - | 66.6 | 0 | - | 100 | 0 | - | 100 | 0 | - | 100 | 50 | - | 50 |
Table 3: Susceptibility pattern of carbapenem resistant clinical isolates
Susceptibility pattern for isolated pathogens from clinical samples was analyzed and the data suggested that all the five pathogens (K. pneumoniae, E. coli, A. baumannii and E. cloacae and S. marcescens) were found susceptible with 100% antimicrobial activity towards Elores which was comparable to colistin to which clinical isolates such as K. pneumoniae (87.3%), E. coli (100%), A. baumannii (100%) and E. cloacae (100%) also displayed high susceptibility. However few studies have shown that colistin is a toxic drug with adverse renal and neurological effects which is not considered safe to the patients.[32] Earlier studies have also documented the greater susceptibility of Elores, the novel antibiotic adjuvant entity, against various clinical pathogens.[22,33,34]
In the present study we observed carbapenem resistant K. pneumoniae exhibited a very high resistance against commonly used drugs such as cefepime+tazobactam (92.1%), piperacillin+tazobactam (98.4%), cefoperazone+sulbactam (100%) and tigecycline (46.1%) [Table 3]. Further, carbapenem resistant E. coli showed second highest resistance against cefepime+tazobactam (84.2%), piperacillin+tazobactam (100%) and cefoperazone+sulbactam (100%). Similarly, carbapenem resistant A. baumannii expressed resistance towards cefepime+tazobactam (66.7%), piperacillin+tazobactam (93.3%) and cefoperazone+sulbactam (40%). Furthermore, least prevalent carbapenem resistant E. cloacae were determined to be 100% resistant against cefepime+tazobactam, piperacillin+tazobactam, cefoperazone+sulbactam. Data also suggested that carbapenem resistant S. marcescens expressed resistance against cefepime+tazobactam (66.6%), piperacillin+tazobactam (100%) and cefoperazone+sulbactam (100%). S. marcescens is inherently resistant to colistin.
The antibiotic resistance rate of clinical pathogens is on a rise in the last few decades. Sharif et al. Demonstrated resistance in E. coli (54%), A. baumannii (37%) and K. pneumoniae (67%) against cefepime.[35] Similarly, Mohammadi and Feizabadi, reported high resistance in gram negative microorganisms against piperacillin+tazobactam (60%).[36] Abdul et al. Have shown the colistin susceptibility among many clinical pathogens [E. coli (96.2%), A. baumannii (92.8%) and K. pneumoniae (93.5%)].[37] While, Kucukates and Kocazeybek, observed that Serratia spp. (67%), Enterobacter spp. (34%), Klebsiella spp. (32%) and E. coli (63%) were displaying resistance towards cefoperazone+sulbactam.[38] Fernandez-canigia and Dowzicky (2012) demonstrated more than 90% of pathogens includes; S. marcescens, A. baumannii and Klebsiella spp. were found to be susceptible towards tigecycline irrespective to ESBL producers or non-producers.[39]
Carbapenems are treated as the last resort to treat serious infected patients. Carbapenem drugs were previously shown to have high antimicrobial activity against ESBL producing organisms[40] but recent reports revealed the non-susceptibility of many pathogens towards carbapenem antibiotics.[41] This may be due to the wide use of these antibiotics as empirical therapy for the treatment of infectious diseases.
Our present data displayed Elores (Antibiotic adjuvant entity) has greater susceptibility against carbapenem resistant isolates which was found unbeatable compared to other antibiotics as discussed above. Penems, BL-BLIs are the commonly used and popular antibiotics and exhibit antimicrobial activity by inhibiting cell wall synthesis but still clinicians face the problem of resistance throughout the world.[42] Many studies documented penems and protein synthesis inhibitors probably have raised resistance among pathogens by over expression of efflux pumps and impairment in the permeability of cell wall[43-45] whereas β-lactam and β-lactamase inhibitors have failed to prevent inactivation via MBLs thus enhancing resistance among pathogens.[36,38,42,46] On the other hand previous studies revealed that Elores (Antibiotic adjuvant entity) has proved its resistance breaking efficacy against most of the pathogens via enhanced penetration through addition of EDTA as adjuvant, biofilm breakage abilities, greater stability and ability to down regulate the over expression of efflux pump.[47-50] Previous data also demonstrated the importance of Elores in the treatment of skin and skin structure infections and bone and joint infections.[51]Therefore, Elores (Antibiotic adjuvant entity) is emerging in the present era as an impactful drug to treat various infectious diseases and combat antibiotic resistance.
Conclusion
In the light of above discussion, it is evident that Antibiotic Adjuvant Therapy scored over protein synthesis inhibitors and β-lactam and β-lactamase inhibitor combinations against carbapenem resistant pathogens due to its resistance breaking mechanisms. In comparison to the antibiotics used for investigating the antibiogram profile of clinical isolates, Elores exhibited prime susceptibility (100% each) which is found comparable to colistin and has again established a mark by getting over antibiotic resistance crisis. Hence Elores is efficient enough to target drug resistant pathogens and indicates a most effective remedy to treat the infections caused by various pathogens and can be considered as empiric choice to spare penems, protein synthesis inhibitors, BL-BLI. Colistin being a highly nephrotoxic drug should be used only when no other choice is available.
REFERENCES
- Gould IM, Bal AM. New antibiotic agents in the pipeline and how they can overcome microbial resistance. Virulence 2013;4:185-91.
- Sengupta S, Chattopadhyay MK, Grossart HP. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front Microbiol 2013;4:47.
- Wright GD. Something new: revisiting natural products in antibiotic drug discovery. Can J Microbiol 2014;60:147-54.
- Piddock LJ. The crisis of no new antibiotics what is the way forward? Lancet Infect Dis 2012;12:249-53.
- Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Clin Opin Pharmacol 2014;18:56-60.
- Centers for Disease Control and Prevention, Office of Infectious Disease Antibiotic resistance threats in the United States 2013.
- Ventola CL. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm Therapeut 2015;40:277-83.
- Read AF, Woods RJ. Antibiotic resistance management. Evol Med Public Health 2014;2014:147.
- Nagaraj S, Chandran SP, Shamanna P, Macaden R. Carbapenem resistance among Escherichia coli and Klebsiella pneumoniae in a tertiary care hospital in south India. Indian J Med Microbiol 2012;30:93-5.
- CDDEP. Resistance Map Washington DC: Centre for Disease Dynamics, Economics and Policy 2015.
- Ahmed GU, Bora A, Hazarika NK, Prasad KN, Randhawa V, Sharma JB, et al. Incidence of bla NDM-1 gene in Escherichia coli isolates at a tertiary care referral hospital in Northeast India. Ind J Med Microbiolo 2013;31:250-56.
- Datta S, Wattal C, Goel N, Oberoi JK, Raveendran R, Prasad KJ, et al. A ten year analysis of multi-drug resistant blood stream infections caused by Escherichia coli and Klebsiella pneumoniae in a tertiary care hospital. Ind J Med Res 2012;135:907-12.
- Grover SS, Sharma M, Chattopadhya D, Kapoor H. Phenotypic and genotypic detection of ESBL mediated cephalosporin resistance in Klebsiella pneumoniae: Emergence of high resistant against Cefepime, the fourth generation cephalosporin. J Infect 2006;53:279-88.
- Parveen RM, Khan MA, Menezes GA, Harish BN, Parija SC, Hays JP, et al. Extended-spectrum β-lactamase producing Klebsiella pneumoniae from blood culture in Puducherry, India. Ind J Med Res 2011;134:392-5.
- Chatterjee D, Sen S, Begum SA, Adhikari A, Hazra A, Das AK, et al. A questionnaire-based survey to ascertain the views of clinicians regarding rational use of antibiotics in teaching hospitals of Kolkata. Ind J Pharmacol 2015;47:105-8.
- Vaja K, Kavathia GU, Goswami YS, Chouhan S. A prevalence study of Acinetobacter species and their sensitivity patter in a tertiary care hospital Rajkot city of Gujrat (India): A hospital based study. J Dent Med Sci 2016;15:54-8.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement. Wayne, PA 19087 USA: CLSI Document M100-S26;2016.
- Akter J, Chowdhury AMMA, Al Forkan M. Study on Prevalence and Antibiotic Resistance Pattern of Klebsiella Isolated from Clinical Samples in South East Region of Bangladesh. American J Drug Disc Dev. 2014;4:73-79.
- Chaudhary BL, Srivastava S, Singh BN, Shukla S. Nosocomial infection due to Multidrug Resistant (MDR) Escherichia coli and Klebsiella pneumoniae in intensive care unit. Int J Curr Microbiol App Sci 2014;3:630-5.
- Chaudhary M, Payasi A. Susceptibility Trend of Drugs Among Metallo β-lactamase Producing Strains of Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa in India 2015;4:2277-4688.
- Darvishi M. Virulence factors profile and antimicrobial resistance of Acinetobacter baumannii strains isolated from various infections recovered from immunosuppressive patients. Biomed Pharmacol J 2016;9:1057-62.
- Kumar M, Chaudhary S, Makkar DJ, Garg N, Chugh S. Comparative antimicrobial efficacy evaluation of a new product Elores against Meropenem of gram negative isolates. Asian J Pharm Clin Res 2015;8:251-4.
- Romanus II, Egwu OA. Analysis of antibiotic susceptibility of Klebsiella pneumoniae isolated from different clinical specimen in enugu state, SENRA Academic Publishers, Burnaby, British Columbia 2011;5:1609-14.
- Shilpa K, Thomas R, Ramyashree A. Isolation and Antimicrobial sensitivity pattern of Klebsiella pneumoniae from sputum samples in a tertiary care hospital. Int J Biomed Adv Res 2016;7:053-7.
- Barakzahi M, Hormozi B, Rashki A, Ghalehnoo ZR. Prevalence of Extended Spectrum β-Lactamase in Klebsiella pneumonia Isolates in a Teaching Hospital of Zahedan City, Iran. Avicenna J Clin Microb Infec 2014;1:e22934.
- Mehta M, Bhardwaj S, Sharma J. Prevalence and antibiotic susceptibility pattern of multi-drug resistant Escherichia coli isolates from urinary tract infection (UTI) patients. Int J Life Sci Pharm Res 2012;2:6-11.
- Kibret M, Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. African Health Sciences 2011;11:S40-S5.
- Jaggi N, Sissodia P, Sharma L. Acinetobacter baumannii isolates in a tertiary care hospital: Antimicrobial resistance and clinical significance. J Microbiol Infect Dis 2012;2:57-63.
- Kaur A, Singh S, Gill AK, Kaur N, Mahajan A. Isolation of Acinetobacter baumannii and it's Antimicrobial Resistance Pattern in an Intensive Care Unit (ICU) of a Tertiary Care Hospital. Int J Contemp Med Res 2016;3:2454-7379.
- Vetter L, Schuepfer G, Kuster SP, Rossi M. A Hospital-wide Outbreak of Serratia marcescens, and Ishikawa’s “Fishbone” Analysis to Support Outbreak Control. Qual Manage Health Care 2016;25:1-7.
- Leski TA, Taitt CR, Bangura U, Stockelman MG, Ansumana R, CooperIII WH, et al. High prevalence of multidrug resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone. BMC Infect Dis 2016;16:167.
- Falagus ME, Kasiakou SK. Toxicity of polymixins, a systematic review of the evidence from old and recent studies. Critical care 2006;10:1-3.
- Makkar DK, Kumar M, Chaudhary S, Goyal S, Aggarwal P, Garg N, et al. Prevalence and Susceptibility Analysis of Gram negative Pathogens. Journal of Pharmacy and Biological Scienc 2015;10:58-65.
- Menon D. A case report of community acquired Pneumonia due to multidrug resistance Pseudomonas aeruginosa treated wit Elores. Int J Med Pharmaceut case reports 2016;6:1-5.
- Sharif MR, Soltani B, Moravveji A, Erami M, Soltani N. Prevalence and Risk Factors associated with Extended Spectrum Beta Lactamase Producing Escherichia coli and Klebsiella pneumoniae Isolates in Hospitalized Patients in Kashan (Iran). Electron Physician 2016; 8: 2081-7.
- Mohammadi MM, Feizabadi MM. Antimicrobial resistance pattern of Gram-negative bacilli isolated from patients at ICUs of Army hospitals in Iran. Iranian J Microbiol 2011;3: 26-30.
- Abdul KG, Vidyalakshmi PR, Jayalakshmi VA, Poojary I. Susceptibility profile of Gram-negative bacteremic isolates to beta lactam-beta lactamase inhibitor agents in comparison to other antibiotics. Indian J Cancer 2014;51:450-52.
- Kucukates E, Kocazeybek B. High resistance rate against 15 different antibiotics in aerobic gram-negative bacteria isolates of cardiology intensive care unit patients. Ind J Med Microbiol 2002;20:208-10.
- Fernández-Canigia L, Dowzicky MJ. Susceptibility of important Gram-negative pathogens to tigecycline and other antibiotics in Latin America between 2004 and 2010. Annals of Clinical Microbiology and Antimicrobials 2012;11:29.
- Paterson DL, Depestel DD. Doripenem. Clin Infect Dis 2009;49:291-8.
- Logan LK, Renschler JP, Gandra S, Weinstein RA, Laxminarayan R. Carbapenem-Resistant Enterobacteriaceae in Children, United States, 1999-2012. Emerging Infect Dis 2015;21: 2014-21.
- Fair RJ, Tor Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect Medicin Chem. 2014;6:25-64.
- Gootz TD, Marra A. Acinetobacter baumannii: An Emerging Multidrug-resistant Threat. Expert Rev Anti Infect Ther 2008;6:309-25.
- Drawz SM, Bonomo RA. Three Decades of -Lactamase Inhibitors. Clin Microbiol Reviews. 2010;23:160-201.
- Singh J, Shukla SK. A New Threat of Bacterial Resistance towards Life Saving Carbapenem Antibiotics. Res J Chemi Sci 2015;5: 85-8.
- Bassetti M, Ginocchio F, Mikulska M, Taramasso L, Giacobbe DR. Will new antimicrobials overcome resistance among Gram-negatives? Expert Rev Anti Infect Ther 2011;9:909-22.
- Chaudhary M, Sudaroli M, Kumar S, Krishnaraju V. Catering ESBL resistance challenge through strategic combination of ceftriaxone, sulbactam and Ethylenediaminetetra acetic Acid. Int J Drug Dev Res 2012;4:72-81.
- Chaudhury M, Kumar S, Payasi A. A novel approach to combat acquired multiple resistance in E. coli by using EDTA as efflux pump inhibitor. J Microb Biochem Technol 2012;4:126-30.
- Chaudhury M, Kumar S, Payasi A. Role of CSE1034 in E. coli biofilm destruction. J Microb Biochem Technol 2013;5:54-8.
- Chaudhury M, Payasi A. Inhibition of metalo beta lactamases by ELORES. J Antimicrob 2013;128:177-82.
- Chaudhary M, Payasi A. Clinical, microbial efficacy and tolerability of Elores, a novel antibiotic adjuvant entity in ESBL producing pathogens: Prospective randomized controlled clinical trial. J Pharmacy Res 2013;7:275-80.