Potential Disadvantages Associated with Treatment of ActiveTuberculosis Using Fixed-Dose Combination: A Review of Literature
2 Department of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
Citation: Iftikha S, Sarwar MR. Potential Disadvantages Associated with Treatment of Active Tuberculosis Using Fixed-Dose Combination: A Review of Literature. J Basic Clin Pharma 2017;8: S131-136
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Background and Objectives: Prevalence of Tuberculosis (TB) is quite high in various regions of the world. Fixed-dose combination (FDC) is highly recommended in various cases like TB, diabetes mellitus, hypertension, etc. Various studies are conducted to assess safety and efficacy of FDC. Likewise, prime focus of present review is to evaluate the potential disadvantages of FDC and compare it with single formulation (SF). Methods: We used a number of electronic databases to identify the relevant published studies which demonstrated the disadvantages regarding fixed dose combination for tuberculosis. Of 204 articles found initially, 143 were selected for additional review. Subsequently, 72 articles were finally selected. Results and Conclusions: Being a disease of poor, several advantages like cost-effectiveness, reduction in pill burden and logistical advantages, etc. can be achieved with FDC. Besides these advantages, there are several disadvantages like poor bioavailability, enzyme level elevation, adverse drug reactions, questionable effectiveness in the absence of Direct Observed Therapy Short course (DOTS) and therapeutic drug monitoring, difficulty in dose adjustment, etc. are associated with FDC. In such consequences there may be the need to reconsider the treatment regimen, otherwise conversion of TB into multi-drug resistant TB (MDR-TB) or extended drug resistant TB (XDR TB) occurs. Hence, it is concluded that FDC is neither superior nor inferior in terms of clinical outcomes and in certain situations the need of SF might not be replaced by FDC.
Keywords
Fixed-dose combination, tuberculosis, disadvantages, single formulation; comparison, bioavailability, pharmacokinetics, rifampicin
Introduction
Tuberculosis (TB) is a widespread disease that effects many living creatures including wildlife, livestock and humans round the globe. [1] TB is the disease of poor which is caused by Mycobacterium tuberculosis and a study revealed that it is seasonal. [2] Globally TB is one of the leading causes of mortalities, especially in adult population. [3] In some regions it’s prevalence is high in males (e.g., India) while in some areas it is prevailing more in females (e.g., Pakistan). [4] Nevertheless, 60% of total TB cases are reported in India, Indonesia, China, Nigeria, Pakistan and South Africa. [5] In heavy TB burden regions like Indonesia TB kills 91,000 people each year which is approximately 7.1% of total deaths. [6,7] In some regions co-morbidities like human immune deficiency virus (HIV) infection is also associated with TB which latter on became epidemic. [8]
On the other hand, it can be preventable. Active lung TB is characterized by cough with sputum and blood in times, weight loss, fever and weakness, etc. The bacilli of TB disperse in air and upon reaching to the alveoli of host, it is attacked by alveolar macrophages and if this causative agent survives from attack then it replicates and diffuses to neighboring cells. [9] Active TB occurs when dynamic equilibrium between host immune system and latent tuberculosis infection (LTBI) breaks. [9] A standard 6 months of therapy is required for the management of active and drug susceptible TB. [10] World Health Organization (WHO), Centers for Disease Control and Prevention (CDC) and International Union Against Tuberculosis and Lung Disease (IUATLD) have been are working on TB since 1994. Three important global health strategies are scaled up by WHO that are; directly observed therapy short course (DOTS), Stop TB and End TB. [11] Fixed-dose combination (FDC) is used in cancer and many neurological disorders [12] to lessen the pill burden and increase patient’s adherence. [13]
In 1999, WHO included FDC in essential drugs list for TB. [14,15] The main aim of WHO is to lessen the mortality rate to 95% and emergence of new TB cases by 90% between 2015 and 2035. [16] A list of advantages associated with FDC is given in Box 1. According to food and drug administration, FDC is “a product composed of any combination of a drug and a device or a biological product and a device or a drug and a biological product or a drug, device, and a biological product”. [17] The current review is subjected to evaluate the need of FDC, scenarios where single formulation (SF) is preferred and the possible disadvantages associated with the treatment of active TB using FDC.
| Advantages related to fixed dose combination | |
|---|---|
| 1. | Reduction in pill burden. [18] |
| 2. | Ease in dispensing. [19] |
| 3. | Simple pharmaceutical management. [20] |
| 4. | Improve adherence and lowers default rate. [21] |
| 5. | Minimum prescription error. [22] |
| 6. | 50% reduction in cost of therapy. [23,24] |
| 7. | Simple treatment plan. [25] |
| 8. | Several logistical advantages like ordering, planning and management of drugs. [24] |
Box 1: Advantages associated with fixed dose combination
Methods
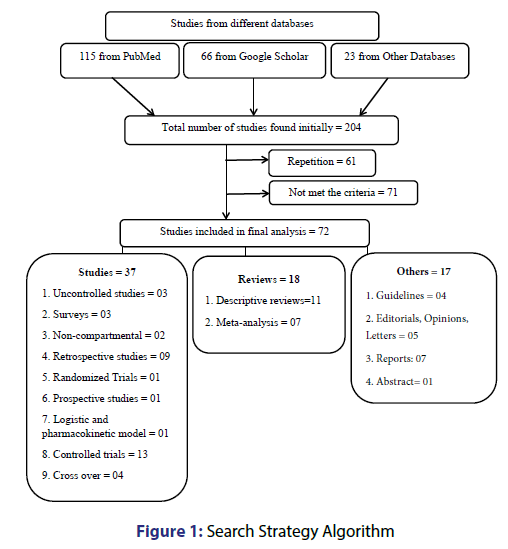
We explored databases (PubMed, Google Scholar, Scopus, ScienceDirect, Embase, ProQuest, EconLit, etc.) from 2010 to February 2017 with these keywords: “fixed dose combination”, “tuberculosis”, “disadvantages” and “adverse drug reactions”, together with names of anti-TB drugs in diverse combinations with BOOLEAN and MeSH search. Further publications were recognized by a manual search of the bibliography and reference section of related papers. Of 204 articles found initially, 143 were selected for further review. Of 143 articles, 72 were finally selected [Figure 1].
Results
Pharmacokinetics of rifampicin
As absorption affects the bioavailability of product, therefore, bioavailability of rifampicin is apocryphal in FDC when compared with SF. Rifampicin being an essential part of FDC, the lower bioavailability of it will cause drug to be less efficacious, therapy failure as well as drug resistance. The suspected factors for lowering of bioavailability are particle size of drug, crystalline form of drug, manufacturing process, characteristics of excipients which leads to breakdown of rifampicin into 3-formyl rifamycin SV at low pH and it is aided by isoniazid. [26]
A two open label, randomized crossover study was conducted on eighteen and twenty healthy Chinese male, with exclusion of patients having drug related allergy or any metabolic disease or drug intake 2 weeks prior to this study, in order to determine relative bioavailability of one 4-drug FDC (4-FDC) and three 2-drug FDC (2-FDC) compared with rifampicin in SF. [27] The three 2-FDC showed lower maximum concentration (Cmax) value and dissolution profile than the SF. So, lower bioavailability and bioequivalence of three 2-FDC in comparison of SF emphasize the need of bioequivalent and bioavailability study of FDC on strict criteria prior to market.
One such study was conducted in South East African country “Malawi” to determine the population pharmacokinetic of adults and children suffering from TB. Candidates were selected and dosed according to their age and weight, as recommended by WHO. In this one compartment model approach, based on first order absorption, study was conducted on total of 165 patients, among them 115 were adults and 50 were children of 4.8 to 87 kg having age between 7 months to 65 years, and those having HIV or risk of HIV were excluded. Relative bioavailability of rifampicin is comparatively low in children when compared with adults and in order to achieve the desired area under the curve (AUC) in children there is need of 15 mg/kg dose increment. So, it was concluded that the WHO recommendations cannot be achievable with currently licensed FDC regimen for TB. [28]
Similarly, relative bioavailability, by a cross-over study, was assessed in Mexican health care system using 3-FDC in comparison of reference products. The results predicted the inferior bioavailability of rifampicin in 3-FDC and strongly suggested that rifampicin containing FDC should only be used after bioavailability testing. [29] Hence, the efficacy of FDC is contentious due to decline rifampicin bioavailability. [30] Likewise, a pharmacokinetic study was conducted in which 8 volunteers were included. It was concluded that when rifampicin is co-administrated with isoniazid, pyrazinamide and ethambutol in FDC then the bioavailability of rifampicin decreases. [31] It was suggested that bioavailability of rifampicin is reduced due to its interaction with isoniazid in acidic pH of gastric media whereas pyrazinamide and ethambutol catalyze this reaction [32] and this problem can be subside by formulating multiparticulate FDC which separately deliver isoniazid and rifampicin. [26] Therefore, bioavailability testing of FDC must be performed to avoid spurious formulations by using strict criteria and it should be within range of 90-111%.Post-prandial absorption
To foreclose and palliate the gastrointestinal related adverse drug reactions (ADR) of anti-TB drugs, patients are directed to take drug with juice or food. [33] But, in a study, Cmax of isoniazid and rifampicin was significantly decline in the presence of food while pyrazinamide and ethambutol were unaffected. [34] The possible reason might be that food increases the gastric emptying time by decreasing motility of stomach. But dissolution and disintegration profile affects absorption in such a manner that if the release rate of formulation is >85% in 10 minutes then fatty food will not have any impact on bioavailability. [35]
An open-label, randomized, cross-over study was conducted in Taiwan [36] which included 16 TB patients and provided them with FDC against TB under DOTS, in order to evaluate the impact of food on pharmacokinetics of FDC and relation between drug concentration and pharmacogenetics. It was concluded that post-prandial serum drug concentration is relatively low as compared to pre-prandial unless dose greater than minimum inhibitory concentration (MIC) is administered. Hence, lower drug concentration leads to treatment failure, so it is recommended to carry out trials for investigating effect of food on patients (having other co-morbidities or not) taking anti-TB drugs and effect of release rate of anti-TB FDC on absorption in presence of food.
Quality of product
Poor quality of product gives poor dissolution profile and lower bioavailabilityResearch data suggest that besides intrinsic factors like absorption there are such extrinsic factors like formulation or bulk material which has significant role in decreasing bioavailability of rifampicin. [37] Similarly, hygroscopic nature of blisters can also deteriorate product quality. Sometimes dissolution test for rifampicin in FDC gives satisfactory result but unable to give compatible bioavailability. It is evident that poor quality of raw material and inadequate manufacturing procedure result in decrease bioavailability of rifampicin in FDC. [38,39] Suspending agent is of key concern when extemporaneous suspension is formed from powder FDC. If poor powder flow properties and sedimentation is associated with suspending agent then it results in poor dissolution profile. [40] Hence, it is recommended that quality during pre and post formulation of FDC must be ensured and only quality certified active ingredients and excipients should be used.
Need of therapeutic drug monitoring
Therapeutic drug monitoring (TDM) is a standard technique in which plasma concentration is measured to evaluate correct dose for a particular patient. But, in all cases merely plasma concentration doesn’t justify patient’s response and treatment outcomes particularly for TB until or unless combine with clinical evaluation and bacteriological data. [41] It was revealed that FDC doesn’t lessen the need of TDM. If the patient is suspected of lower plasma concentration then it will evoke the need of TDM. But higher toxicity risk must be kept in mind in case of pyrazinamide. As it is not feasible to perform TDM on every patient, studies suggested to perform TDM for patient with slow sputum conversion, those having high risk of co-morbidities and drug-drug interactions. [42] Emphasis was made on need of TDM of rifampicin as it shows concentration dependent killing of causative agent of TB, therefore, it’s low level may cause failure of therapy or resistance or delay response. [43]Effect of co-morbidities on absorption
There are several co-morbidities like diabetes mellitus (DM), HIV infection, etc. that are associated with TB. The TB is thought to occur 17 times more in patients suffering from HIV. [44] Serum concentration of anti-TB FDC drugs are significantly lower in HIV/TB co-infected patients. [45] In a study incomprehensive results of lower absorption of isoniazid and rifampicin were observed in HIV/TB co-infected patients of Kampala, Uganda. [46] If the CD4 cell count is <200/mm3 in HIV patient then absorption of FDC decreased significantly. [47] Simultaneous disease states cause the slow response of patients towards therapy. [48] It is evident that if patient have symptoms of acquired immune deficiency syndrome (AIDS) then poor absorption of anti-TB drugs occur. [39]
Treatment regimen for TB is same in both HIV positive and HIV negative patients. [49] But HIV causes poor treatment outcomes of anti-TB drugs. Since this virus impairs immune system of the host therefore adverse drug reactions (ADRs) due to anti-TB drugs are more common where there is an increase prevalence of HIV [39] and also in a retrospective study severe ADRs were seen in patients having low CD4 cell count (130 and 259 cells/µL). [50] A cross-sectional study was conducted at Gondar University Hospital, Northwest Ethiopia revealed that besides ADRs, treatment outcome was also found lower with current WHO prescribed regimen in HIV/TB co-infection. [51] It is recommended that frequent HIV diagnostic tests must be performed for newly diagnosed TB patients in high HIV/TB burden areas and protocols for good treatment outcome must be designed. Furthermore, TDM is necessary to monitor low serum drug concentration as well as malabsorption and, if needed, therapy must be switched towards higher doses.
Altered level of liver enzymes
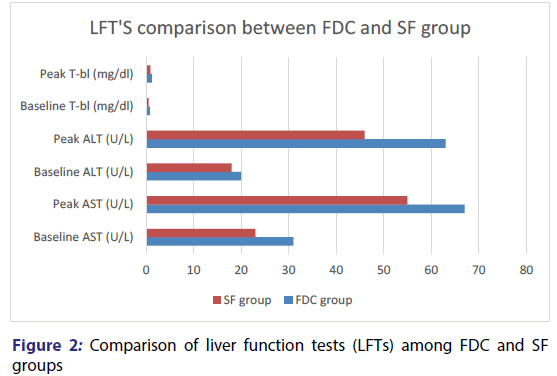
When FDC compared with SF then elevation of several liver enzymes was reported in FDC group, like aspartate aminotransferase (AST), alanine aminotransferase (ALT) along with total bilirubin (T-bl) which may lead to asymptomatic jaundice and drug induced hepatitis. [52] An open ended, prospective and randomized trial [23] was carried out in E-DA teaching hospital of Taiwan from October 2008 to November 2009. Patients of ≥ 18 years of age were included and exclusion criteria was based on abnormal baseline functioning of liver, life expectancy of <6 months and received anti-TB regimen in past. Candidates were randomly placed in FDC and SF groups. To observe response of therapy WHO guidelines were followed. Hence, comparison of FDC with SF demonstrated that ALT, AST and t-bl level is significantly high [Figure 2].
Risk of toxicity of anti-TB drugs becomes higher when AST>3 times upper level of normal in the presence of symptoms or >5 times upper limit of normal in the absence of symptoms. Toxicity of mild level occurs if AST and ALT<5 times the upper limit of normal. Moderate level toxicity can be seen if AST and ALT levels rise by 5-10 times the normal and if >10 times the normal then toxicity is considered to be severe. With any of the stated increment all of the anti-TB therapies have to be stopped. The drugs can be reintroduced when abnormality comes to an end in such a manner that least likely causative agent should be reintroduced first. If toxic level will not be reoccurred then the final drug considered as the causative agent and shouldn’t be reintroduced in the therapy. [53] It is difficult to find relation between specific chemical entity and the ADR. [54] It is recommended that if elevated LFTs are obtained then there is a need of conducting serology test for viral infection as a confirmation test for acute viral hepatitis [55] and limited stock of SF must be available in order to combat such conditions. [52]
Hematology related side effects
Hematology related side effects are more common with FDC of anti-TB drugs because doses in FDC are different from that of SF. These side effects include; urticaria, thrombocytopenia and leucopenia, etc. One such retrospective study was conducted in which a total of 560 patients were divided into two groups. Group 1 received FDC for 2 months of TB treatment and group 2 received SF during the treatment. Urticaria, thrombocytopenia and leucopenia were more in patients treated with FDC due to significant difference in isoniazid and rifampicin in both groups. [56] Similarly, various other studies also reported hematologic side effects like urticaria with FDC. [57,58] It is recommended that health care providers must educate the patients about the possible side effects of the therapy and patients should seek medical guidance immediately in such situations.
Need of direct observed therapy
DOTS means that patient intakes medicines in the presence of supervisor which is usually health care provider, for at least two months of the therapy. [59] Risk of poor adherence is there if DOTS is not followed because poor adherence of patient towards medicine is the result when patient is careless or forgets to take medicine, when patient feels better or worst then he stops taking medicine. [60] FDC improves patient adherence towards treatment by the introduction of patient’s kits but evidences supports the fact that FDC doesn’t lessen the need of DOTS. [61] Therefore, several potential advantages of FDC cannot be achieved without following DOTS. [24,62] Hence, it is recommended that DOTS must be followed in patients with newly diagnosed TB and default cases.
Resistance
Wrong time medication error can be the cause of resistance in such a way that when the FDC is taken on irregular interval of time then chances of resistance becomes several folds. [10] CDC, in the guidelines of 2013, recommends that there is an increased risk of rifampicin resistance if rifampicin containing regimen is administered once, twice, or thrice weekly in patients with advanced HIV (CD4 cell count <100 cells/mm3) and in case of high load of Mycobacterium tuberculosis. Therefore, anti-TB drugs especially rifampicin must be administered 5 to 7 days per week in HIV/TB co-infection for first 2 months of treatment. [63] Like SF, chances of resistance are still there if FDC are given without any supervision and poor quality of FDC leads to multi-drug resistance TB (MDR-TB). [39] If FDC is used occasionally then it might be the cause of resistance. If DOTS is adopted then chances of drug resistance can be minimized. [24,64] Hence, it is recommended to follow proper DOTS with standard quality FDC.Relapse rate
Relapse or recurrence occurs in patients who are unresponsive towards the anti-TB drugs. [47] One of the major issues is that relapse rate doesn’t minify with FDC. Probably poor bioavailability of drugs and poor clinical practices can be the possible reasons. One such retrospective study was conducted in the Department of Pneumology la Rabta to check the efficacy, tolerance and relapse between FDC and SF in patients of pulmonary tuberculosis with first attack. It was concluded that there was no significant difference between two groups in terms of relapse rate. [57] But a contradicted result was shown in a systematic review that there was more chances of relapse with FDC than the SF because of poor bioavailability of drugs in FDC. [65] Similarly, two studies gave the same result of high relapse rate and treatment failure with FDC. [66,67] Therefore, there is a need to improve clinical malpractice in high TB burden areas along with bioavailability issues.
Non-superiority of fixed dose combination
A meta-analysis provided evidences that there is no significant difference in treatment with FDC over SF. [42] Factors like poor quality of rifampicin and quantity of isoniazid make FDC non-superior [68] but it’s potential advantages [Box 1] make it non-inferior than SF. A randomized controlled trial [54] was conducted in which register of Cochrane Infectious Disease Group Specialized Register, Medline, Embase, Lilacs, the metaRegister of Controlled Trials and the World Health Organization International Clinical Trials Registry Platform (WHO-ICTRP) was searched till 20th November 2015 without language barrier and it was found that there is no significant difference between FDC and SF with respect to acceptability, safety and efficacy. Likewise, difference in cure rate was also determined between these two regimens. [69] Similarly, two systematic reviews were carried to demonstrate treatment outcomes between FDC and SF, but no improved treatment outcome with FDC was obtained. [65,70] As FDC is neither superior nor inferior so, clinical trials on large scale must be conducted to prove or nullify this fact.
Patient with higher body mass index
Over-weight and obesity is prevailing globally especially in poor socio-economic regions where the risk of TB is also very high. [71] Dose individualization is difficult with FDC [22] as the drug metabolism is altered with increased body mass index (BMI) or obesity. In a study [72] a case was reported of 36 years old Algerian man who was 92 kg of weight with a BMI of 28.4 kg/m2, as he was diagnosed with positive for Mycobacterium tuberculosis complex, he was prescribed with FDC of isoniazid, rifampicin and pyrazinamide (6 tablets daily), latter on he was diagnosed with Mycobacterium bovis. As Mycobacterium bovis is resistant to pyrazinamide, so prescriber switched to isoniazid and rifampicin containing FDC of maximum prescribed dose. After 3 months of treatment patient didn’t get better. Due to poor clinical outcome, when TDM was conducted then it was found that there is sub-therapeutic concentrations of drugs at this regimen. So, the need of higher doses with single dose regimen with TDM is justified and is recommendable, otherwise poor clinical outcome may leads to therapy failure.Preference of single formulation over fixed dose combination
In certain conditions, when there is the need of dose adjustment then FDC proves to be an untrammeled. [53] SF is also preferred when ADR arise or LFTs elevate with FDC. Therefore, it is recommended to keep limited stock of SF along with the procurement of FDC.
Limitations
Due to lack of data our review couldn’t access the logical reasons of pharmacokinetic variability of FDC in patients having TB/HIV, TB/DM or any other co-morbidities. Similarly, relation of immunity suppression and lower absorption of rifampicin in FDC couldn’t be justified.
Conclusion
There are several advantages [Box 2] associated with FDC, on the basis of which WHO justified the need of it in TB treatment, cannot be denied. It is concluded that slow response of therapy, treatment failure, higher ADRs and development of MDR-TB or XDR-TB can occur with FDC because of poor absorption, poor bioavailability, suppressed immunity, inferior quality of drug and post-prandial drug administration, etc. So, in comparison with SF it is found that FDC is neither superior nor inferior. Therefore, it can’t takeover SF completely especially in chronic diseases like TB and limited stock of SF anti-TB drugs must be available all the time in patient-care settings.
| Disadvantages associated with fixed dose combination | |
|---|---|
| 1. | Altered pharmacokinetics of rifampicin. |
| 2. | Altered post prandial absorption. |
| 3. | Consequences of quality on success rate. |
| 4. | Prerequisite of TDM. |
| 5. | Effect of co-morbidities on absorption. |
| 6. | Worse treatment outcome in HIV/TB. |
| 7. | Abnormal liver function tests. |
| 8. | Hematologic side effects. |
| 9. | Undeniable requisition of DOTS. |
| 10. | Higher risks of resistance. |
| 11. | Non-superiority of FDC over SF. |
| 12. | Necessity of SF. |
| 13. | Troublesome dose adjustment. |
Box 2: Summary of potential disadvantages associated with fixed dose combination
Ethics approval
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
REFERENCES
- Patterson S. Social and environmental factors affect tuberculosis related mortality in wild meerkats. J Anim Ecol 2017.
- Khaliq A, Batool SA, Chaudhry MN. Seasonality and trend analysis of tuberculosis in Lahore, Pakistan from 2006 to 2013. J Epidemiol Glob Health 2015;5:397-403.
- Bleed D, Watt C, Dye C. Global Tuberculosis Control: WHO Report: World health organization (WHO) 2000.
- Codlin AJ. Short report: Gender differences in tuberculosis notification in Pakistan. Am J Trop Med Hyg 2011; 85:514-7.
- World Health Organization, Global tuberculosis report 2016.
- Organization WH, Global tuberculosis control: epidemiology, planning, financing: WHO report 2009.
- Factor that influence treatment adherence of Tuberculosis patients living in Java, Indonesia. Patient Prefer Adherence 2009;3:231-8.
- Sileshi B. Predictors of mortality among TB-HIV Co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC infect diseases 2013;13:297.
- Giovanni Delogu MS, Giovanni Fadda. The Biology of Mycobacterium Tuberculosis Infection. Mediterr J Hematol Infect Dis 2013;5.
- Yew WW, Lange C, Leung CC. Treatment of tuberculosis: update 2010. Eur Respiratory Soc 2011.
- Sotgiu G, Sulis G, Matteelli A. Tuberculosis-a World Health Organization Perspective. Microbiol Spectr 2017; 5.
- Sahota T, Della Pasqua O. Feasibility of a fixed-dose regimen of pyrazinamide and its impact on systemic drug exposure and liver safety in patients with tuberculosis. Antimicrobial Agents Chemother 2012;56:5442-9.
- Furin J. ‘I'm fed up': experiences of prior anti-tuberculosis treatment in patients with drug-resistant tuberculosis and HIV. The Internation J.Tuberculo and Lun Disease 2014;18:1479-84.
- World Health Organization, WHO drug information 1999;249-62.
- World Health Organization, The use of essential drugs. Ninth report of the WHO Expert Committee (including the revised Model List of Essential Drugs) 2000.
- World Health Organization. WHO End TB Strategy 2017. Available from: http://www.who.int/tb/strategy/end-tb/en/.
- Gautam CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol 2008;65:795-6.
- Christian Lienhardt M. Efficacy and Safety of a 4-Drug Fixed-Dose Combination Regimen Compared With Separate Drugs for Treatment of Pulmonary Tuberculosis The Study C Randomized Controlled Trial. JAMA 2011;305.
- Partnership ST. Global drug facility. Frequently asked questions about the 4-drug fixed-dose combination tablet recommended by the World Health Organization for treating tuberculosis. September 2002;24.
- Lima GC. safety of a four-drug fixed-dose combination regimen versus separate drugs for treatment of pulmonary tuberculosis: a systematic review and meta-analysis. Braz J Microbiol 2016.
- Braga JU, Trajman A. Effectiveness of RHZE-FDC (fixed-dose combination) compared to RH-FDC + Z for tuberculosis treatment in Brazil: a cohort study. BMC Infect Dis 2015;15:81.
- Lloret-Linares C. Inadequate therapeutic response to a recommended antituberculosis fixed-dose combination regimen in an overweight patient with Mycobacterium bovis infection. Ann Pharmacother 2013;47:e4.
- Wu JT. Comparison of the safety and efficacy of a fixed-dose combination regimen and separate formulations for pulmonary tuberculosis treatment. Clinics (Sao Paulo) 2015; 70:429-34.
- Monedero I, Caminero J. Evidence for promoting fixed-dose combination drugs in tuberculosis treatment and control: a review Unresolved issues. The Internation J. Tuberculo & Lun Dis 2011;15:433-9.
- Bangalore S. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713-9.
- Du Toit LC, Pillay V, Danckwerts MP. Tuberculosis chemotherapy: current drug delivery approaches. Respir Res 2006;7:118.
- Zhu H. Relative bioavailability of rifampicin in four Chinese fixed-dose combinations compared with rifampicin in free combinations. Chin Med J (Engl) 2015;128:433-7.
- Schipani A. A simultaneous population pharmacokinetic analysis of rifampicin in Malawian adults and children. Br J Clin Pharmacol 2016;81:679-87.
- Milan-Segovia RC. Relative bioavailability of rifampicin in a three-drug fixed-dose combination formulation. Int J Tuberc Lung Dis 2010;14:1454-60.
- Shin HJ, Kwon YS, Treatment of Drug Susceptible Pulmonary Tuberculosis. Tuberc Respir Dis (Seoul) 2015;78:161-7.
- Chandra I. Bioavailability of rifampicin following concomitant administration of ethambutol or isoniazid or pyrazinamide or a combination of the three drugs. Ind.J.Med Res 2003;118:109.
- Mathew JL. Fixed dose drug combination for treatment of tuberculosis. Ind.Pediatr 2009;46:877-80.
- Requena-Méndez A. Pharmacokinetics of rifampin in Peruvian tuberculosis patients with and without comorbid diabetes or HIV. Antimicrobial agents and chemother 2012;56:2357-63.
- Tostmann A. Pharmacokinetics of first-line tuberculosis drugs in Tanzanian patients. Antimicrob Agents Chemother 2013;57:3208-13.
- Panchagnula R. In vitro evaluation of food effect on the bioavailability of rifampicin from antituberculosis fixed dose combination formulations. Farmaco 2003;58:1099-103.
- Lin HC. Impact of food intake on the pharmacokinetics of first-line antituberculosis drugs in Taiwanese tuberculosis patients. J Formos Med Assoc 2014;113:291-7.
- Agrawal S. Bioequivalence trials of rifampicin containing formulations: extrinsic and intrinsic factors in the absorption of rifampicin. Pharmacol Res 2004;50:317-27.
- Acocella G. Human bioavailability studies. Bull Int Union Tuberc Lung Dis 1989;64: 38-40:40-2 discussion.
- Blomberg B.The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull World Health Organ 2001;79:61-8.
- Danckwerts MP, Ebrahim S, Pillay V. Pharmaceutical formulation of a fixed-dose anti-tuberculosis combination. Int J Tuberc Lung Dis 2003;7:289-97.
- Peloquin C, The Role of Therapeutic Drug Monitoring in Mycobacterial Infections. Microbiol Spectr 2017;5.
- Zuur MA. Fixed-dose combination and therapeutic drug monitoring in tuberculosis: friend or foe? Eur Respir J 2016;48:1230-3.
- Chawla PK. Importance of Therapeutic Drug Monitoring of Rifampicin. J Assoc Physicians Ind 2016;64:68-72.
- Hawn TR. Tuberculosis vaccines and prevention of infection. Microbiol Mol Biol Rev 2014;78:650-71.
- Bhatt NB. Pharmacokinetics of rifampin and isoniazid in tuberculosis-HIV-coinfected patients receiving nevirapine-or efavirenz-based antiretroviral treatment. Antimicrobial agents and chemother 2014;58:3182-90.
- Sekaggya Wiltshire C. Low isoniazid and rifampicin concentrations in TB/HIV co-infected patients in Uganda. J. Internation AIDS Society 2014;17.
- Suryanto A. Is there an increased risk of TB relapse in patients treated with fixed-dose combination drugs in Indonesia? The international journal of tuberculosis and lung disease 2008;12:174-9.
- Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 2002;62:2169-83.
- Swaminathan SC, Padmapriyadarsini, Narendran G. HIV-associated tuberculosis: clinical update. Clin Infect Dis 2010;50:1377-86.
- Marks DJ. Adverse events to antituberculosis therapy: influence of HIV and antiretroviral drugs. Int J STD AIDS 2009;20:339-45.
- Sinshaw Y. Successful TB treatment outcome and its associated factors among TB/HIV co-infected patients attending Gondar University Referral Hospital, Northwest Ethiopia: an institution based cross-sectional study. BMC Inf Dis 2017;17:132.
- Organization WH, Initiative ST. Treatment of tuberculosis: guidelines: World Health Organization 2010.
- World Medical Association, A tuberculosis refresher course for physicians: World Medical Association 2007;95.
- Gallardo CR. Fixed-dose combinations of drugs versus single-drug formulations for treating pulmonary tuberculosis. Cochrane Database Syst Rev 2016;CD009913.
- Jeong I. Drug-induced hepatotoxicity of anti-tuberculosis drugs and their serum levels. J.Korean med sci 2015;30:167-72.
- Majdoub S. Antituberculosis fixed multi-dose combination and single drug therapy in active tuberculosis: What is about drug hypersensitivity reactions? Eur Resp Soc 2016.
- Toujani S. Contribution of fixed-dose combinations in the treatment of tuberculosis. Tunis Med 2016;94:401-5.
- Kotti A. Antituberculosis fixed multi-dose combination and single drug therapy in active tuberculosis: What is the benefit? Eur Resp Soc 2011.
- Newell JN. Control of tuberculosis in an urban setting in Nepal: public-private partnership. Bull World Health Organ 2004;82:92-8.
- Nezenega ZS, Gacho YH, Tafere TE. Patient satisfaction on tuberculosis treatment service and adherence to treatment in public health facilities of Sidama zone, South Ethiopia. BMC Health Serv Res 2013;13:110.
- Joshi JM, Tuberculosis chemotherapy in the 21 st century: Back to the basics. Lun Ind 2011;28:193.
- Phanouvong S. Operational guide for national tuberculosis control programmes on the introduction and use of fixed-dose combination drugs 2002.
- Control CD, Prevention, Managing drug interactions in the treatment of HIV-related tuberculos 2013.
- Nolan CM, Taylor Z, Blumberg HM, Failure to mention fixed-dose drug combinations in the ATS/CDC/IDSA tuberculosis control statement. Ame. j.res & crit care med 2006;173:684a-5.
- Albanna AS. Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. Eur Res.J 2013;42:721-32.
- Girling D, Teo S. Assessment of a daily combined preparation of isoniazid, rifampin, and pyrazinamide in a controlled trial of three 6-month regimens for smear-positive pulmonary tuberculosis. American J. Res & Crit Care Med1991; 143:707-12.
- Teo S. Assessment of a combined preparation of isoniazid, rifampicin and pyrazinamide (Rifater®) in the initial phase of chemotherapy in three 6-month regimens for smear-positive pulmonary tuberculosis: a five-year follow-up report. The International J.Tuberculo and Lung Dis 1999;3:26-132.
- Nunn A. Results at 30 months of a randomised trial of FDCs and separate drugs for the treatment of tuberculosis. The International J.Tuberculo and Lung Dis 2014;18:1252-4.
- Ferreira ACG. Clinical treatment outcomes of tuberculosis treated with the basic regimen recommended by the Brazilian National Ministry of Health using fixed-dose combination tablets in the greater metropolitan area of Goiânia Brazil. J. Brasileiro de Pneumologia 2013;39:76-83.
- Mahadeo R, Gounder S, Graham SM. Changing from single-drug to fixed-dose combinations: experience from Fiji. Pub Health Action 2014;4:169-73.
- McLaren L. Socioeconomic status and obesity. Epidemiolo Rev 2007;29:29-48.
- Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010;49:71-87.