Novel Approaches in Formulation and Drug Delivery using Contact Lenses
- *Corresponding Author:
Date of Received: 18-04-2011
Date of Modified: 05-05-2011
Date of Accepted: 10-05-2011
Available Online: 15-05-2011
Abstract
The success of ocular delivery relies on the potential to enhance the drug bioavail- ability by controlled and extended release of drug on the eye surface. Several new ap- proaches have been attempted to augment the competence and diminish the intrinsic side effects of existing ocular drug delivery systems. In this contest, progress has been made to develop drug-eluting contact lens using different techniques, which have the potential to control and sustain the delivery of drug. Further, the availability of novel polymers have facilitated and promoted the utility of contact lenses in ocular drug delivery. Several research groups have already explored the feasibility and potential of contact lens using conventional drugs for the treatment of periocular and intraocular diseases. Contact lenses formulated using modern technology exhibits high loading, controlled drug release, apposite thickness, water content, superior mechanical and optical properties as compared to commercial lenses. In general, this review discus various factors and approaches designed and explored for the successful delivery of ophthalmic drugs using contact lenses as drug delivery device.
Keywords
Contact lenses, ocular delivery, drug loading, controlled release
Abbreviations
β-CD beta-cyclodextrin
CDs Cyclodextrins
EGF Epidermal growth factor
GMA Glycidyl methacrylate
HEMA Hydroxyethyl methacrylate
ODDS Ophthalmic drug delivery systems.
PDMS Polydimethylsiloxane
pHEMA Poly-hydroxyethyl methacrylate
scCO2 Super critical carbon dioxide
SCLs Soft contact lenses
SSI Solvent impregnation
Tg Glass transition temperature
Introduction
Several eye diseases like cataract, age-related macular degeneration, diabetic retinopathy, glaucoma etc have reported to affect several millions of the world population [1,2]. Existing treatment modalities include topical, introaocular and systemic delivery. The intraocular and systemic therapy is not widely used due to their intrinsic limitations. However, the topical route is the most appropriate therapy for local and controlled delivery of bioactive and therapeutic molecules to the eye, as it offers higher patient compliance, better proximity to the infected site and avoids potential systemic side effects as well [3,4]. Among the topical formulations, conventional topical eye drops is well accepted and represents ~70-90% of the marketed formulations. However, the success of topical therapy is limited by several issues such as low bioavailability (>1%), short residence time, low corneal permeability, effective removal mechanisms and rapid loss through nasolacrimal drainage [5,6]. Further, frequent administration of high concentration of medication from topical eye drops is extravagant and diminishes the patient compliance [7,8]. Thus, the major challenge in ocular drug delivery remains the controlled and extended delivery of medication in a therapeutically relevant concentration. Hence, the quest for developing systems that can deliver effective concentration of drugs in a comprehensive way was initiated [9]. Several approaches were designed and explored to augment the therapeutic efficiency of topically applied ocular formulations and there by surmount the inherent limitations. Extensive studies were carried out to develop efficient ophthalmic drug delivery systems (ODDS) particularly to improve therapeutic duration, targeting and compliance in a more predictable and reproducible way [10-12]. Consequently, few products including contact lenses, have been launched in the market while many others are in pre-clinical and clinical stages. These includes polymeric/mucoadhesive formulations, hydrogels, in situ gelling systems, colloidal systems, ocular inserts, collagen shields, poly(tetrafluoroethylene) strips, intravitreal implants, punctual plugs etc. [13-15].
Commercial contact lenses are competent to enhance the bioavailability however could not find their way in prolonging the drug delivery. The potential application of contact lens as ODDS was first described by Sedlacek [16]. Contact lenses are particularly attractive for ODDS as these significantly increase the residence time (typically days) of the drug in the eye, high degree of comfort and enhances the drug bioavailability considerably. Survey of literature suggests that the soft contact lens receives much attention in recent days as drug delivery device. Approaches such as incorporation of binding agents by molecular imprinting, entrapment of vesicular systems into the lens matrix are found to be promising. Various research groups have focused on the development of contact lenses which can extend the residence time in eye and thereby control the drug release for an extended period of time. This review discusses the recent developments in contact lens drug delivery for the effective and efficient delivery of drug to the eye.
Snags in Ophthalmic Drug Delivery
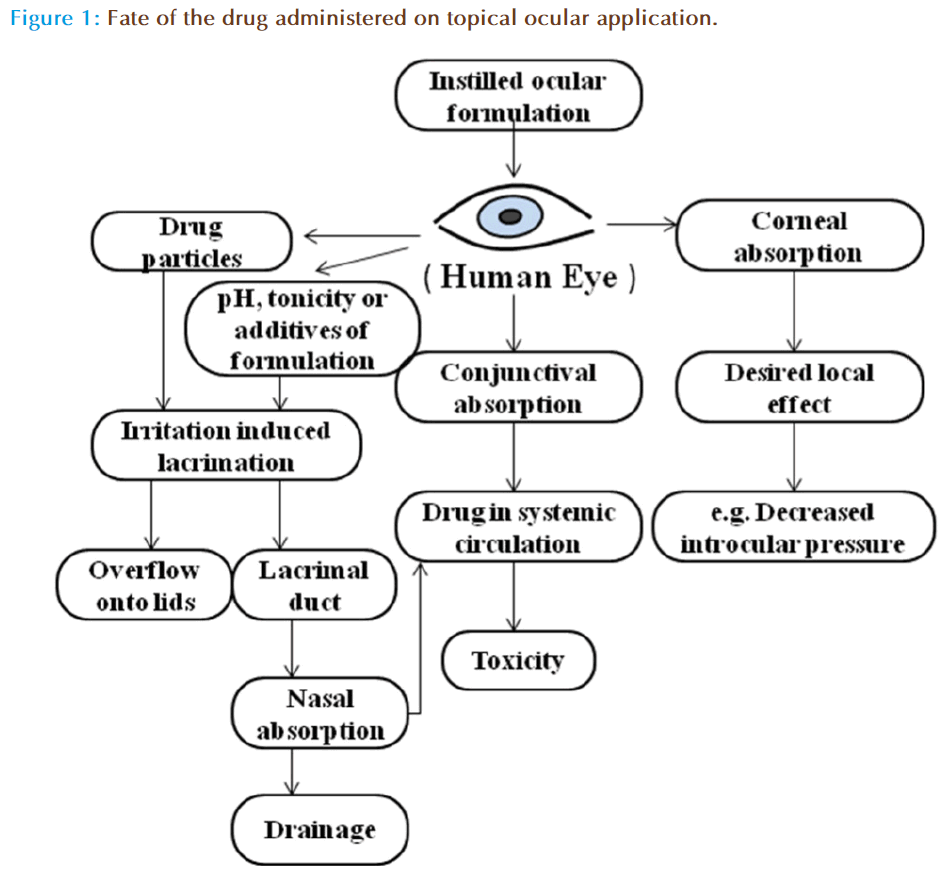
There are several physiological barriers to diffusion and productive absorption of topically applied drugs in the precorneal and corneal spaces (Figure 1). The precorneal restrictions responsible for poor ocular bioavailability of conventional ophthalmic dosage forms are solution drainage, lacrimation, tear dilution, tear turnover and conjunctival absorption. On instillation the drug mixes with the tear fluid and leaves the precorneal area within 2 min, is the key factor in reducing the contact time of drug with cornea and consequently ocular bioavailability of topical dosage forms. The drug unabsorbed by the cornea is either absorbed by the conjunctiva or flows through the upper and the lower canaliculi into the lacrimal sac [17]. The conjunctiva possesses a relatively large surface area, 5 times the surface of cornea making the loss significant. Alternatively, tears dilute the remaining drug in the cul-de-sac which subsequently reduces the transcorneal flux. The drug entity, pH, tonicity of the dosage forms as well as formulation adjuvants can stimulate tear production which may eventually dilute the formulation. Further, the drug containing tear fluid is carried from lacrimal sac into nasolacrimal duct, and is absorbed into the systemic circulation. However, the absorption of drugs into bloodstream leads to depletion of chemical moieties and its existence in systemic circulation could leads to undesirable side effects. For example, ß-blockers such as timolol, used in the treatment of wide-angle glaucoma, have a deleterious effect on heart. Thus, new ODDS such as contact lenses are primarily aimed to increase the residence time of the drug in the corneal region, and limiting the drug loss by drainage, lcarimation or conjunctival absorption.
Advantages
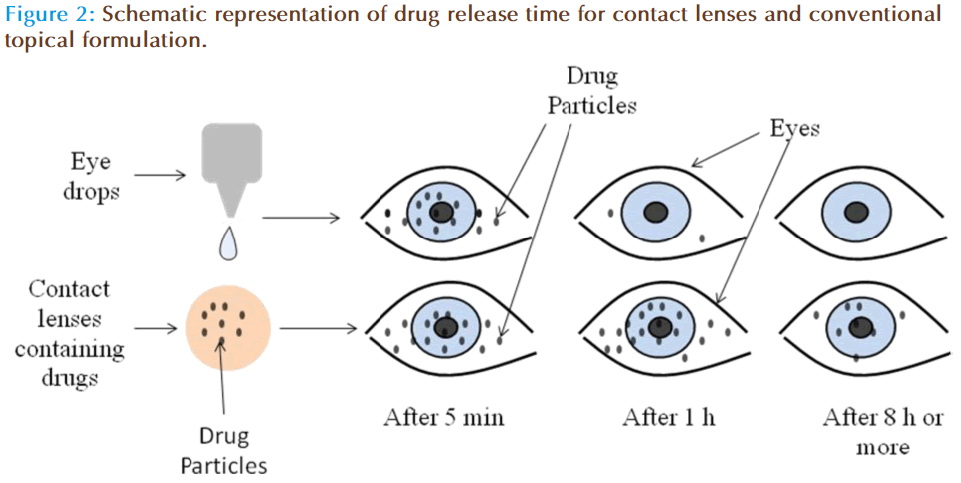
Several benefits including increased ocular bioavailability, prolonged residence time, improved patient compliance, higher efficiency and low side effects are accounted for contact lenses in the literature, compared to conventional dosage forms (Figure 2). The soft contact lenses (SCLs) possess multiple advantages such as ease of application, convenient for long term therapy due to their excellent biocompatibility, provides continuous drug delivery, unproductive systemic absorption, higher patient compliance etc. Indeed, medicated SCLs may combine the role of ODDS with the correction of refractive deficiencies. However, contact lens loaded by soaking technique possess few confines such as limited drug incorporation in lens matrix, incompetent to offer slow and extended drug release, wastage of large fraction of drug during loading procedure etc. [18]. However, recent developments in techniques such as molecular imprinting and supercritical solvent impregnations have surmounted all these issues [19].
Polymers in Ocular Contact Lenses
The most popular type of ophthalmic lens is soft contact lens made of thermo-set polymer hydrogels. These gels possess three dimensional, amorphous networks with cross-links, similar to hard contact lens polymers, and are typically produced by cast molding or spin cast method [20]. The reason for the softness of the lens is principally because these polymers exist above its glass transition temperature (Tg). Otto Wichterle discovered poly-hydroxyethyl methacrylate (pHEMA) a hydrogel, in 1961and created the world’s first soft lens. In1971, FDA approved hydroxyethyl methacrylate (HEMA) based contact lenses for daily wear. In 1999, silicone hydrogels become available which are used to form the extended wear contact lenses that are permeable to oxygen. Although it provides the oxygen permeability, the presence of silicone makes the lens surface highly hydrophobic and less wettable. This frequently results in discomfort and dryness during lens wear. Usually hydrogels are incorporated to compensate the hydrophobicity, however, the lens surface remains hydrophobic. Hence, the lens surface is generally modified by plasma treatments which can alter the hydrophobic nature of the lens. On the other hand, few lens types have incorporated internal rewetting agents to ensure the hydrophilicity of the lens surface. A third process uses longer backbone polymer chains that results in less cross linking and increased wetting without surface alterations. Several companies are fabricating contact lenses including four major manufacturers from the United States (Table 1). At present, there are seven different disposable silicone hydrogel lens materials commercially available in the United States which include balafilcon A (PureVision, B&L), lotrafilcon A (Night & Day, CIBA Vision), galyfilcon A (Acuvue Advance, Vistakon), lotrafilcon B (O2Optix, CIBA), senofilcon A (Acuvue Oasys, Vistakon), comfilcon A (Biofinity, CooperVision) and enfilcon A (Avaira, CooperVision). The lenses are prepared by different strategies, for instance, balafilcon A is a homogeneous combination of a tris[trimethylsiloxy] silylpropyl-methacrylate (TRIS) monomer derivative that is copolymerized with the hydrophilic hydrogel monomer N-vinyl pyrrolidone. Lotrafilcon A involves polymerizing unmodified TRIS and macromers with silicone elastomer sequences interspersed with hydrophilic polyethylene glycols, and each lens is treated to create a hydrophilic surface. Both galyfilcon and senofilcon are differentiated from the first-generation silicone hydrogels (Balafilcon A and lotrafilcon A) because no surface treatment is applied. Instead, in both the cases a long-chain, hyhigh- molecular-weight polyvinyl pyrrolidone was incorporated, which serves as an internal wetting agent for galyfilcon A (Hydraclear) and senofilcon A (Hydraclear Plus). Comfilcon A (Biofinity, CooperVision) and enfilcon A (Avaira, CooperVision) are the latest silicone hydrogel entries. Both uses a unique long-chain siloxane macromer combined with other components to result in a lens that features high oxygen permeability and a relatively low modulus [21]. These materials are inherently wettable and no internal wetting agent or surface treatment is required.
| Extended wear lenses | Manufacturer | Application period | Material | % Water content | Lens permeability (barrer) Dk |
|---|---|---|---|---|---|
| Night & Day | Ciba Vision | 30 days | Lotrafilcon A | 24 | 140 |
| Pure Vision | Bausch & Lomb | 30 days | Balafilcon A | 36 | 99 |
| O2 Optix | Ciba Vision | 7 days | Lotrafilcon B | 33 | 110 |
| Acuvue Oasys | Jonson and Jonson | 7 days | Galyfilcon A | 36 | 103 |
| Biofinity | CooperVision | 7 days | Comfilcon A | 48 | 128 |
Table 1: FDA approved silicon c ontact lenses and their manufacturers.
Factors Affecting Drug Delivery
Transparency of the lens
Transparency of the formulated contact lens is a key factor affecting the drug delivery, and cannot be compromised due to the incorporation of drug particles or additives. The novel approaches have enabled to formulate and incorporate materials in contact lenses which demonstrated good transparency. For instance, contact lenses formulated with advanced techniques such as molecular imprinting and supercritical solvent approaches, loaded liposomes and microemulsions, exhibited good transparency [22,23].
Oxygen permeability
Human eye does not receive adequate blood flow to supply the eye with enough oxygen, or to remove enough carbon dioxide. It mainly relies on its exposure to the air for oxygen supply. Hence, the prepared lenses should allow free transfer of oxygen to the eyes and any low oxygen transfer eventually causes severe side effects [24]. The lens permeability (Dk) is the product of the diffusivity (D) and the oxygen partition coefficient (k), and it is typically expressed in units of 10-11 (cm2/s)*(mlO2/(ml mmHg)) or 1011 mlO2 cm/(s cm2 mmHg), which is also referred as barrer or Fatt. The oxygen permeability is an intrinsic property of a material to transport oxygen through its bulk and is independent of thickness. The oxygen transmissibility refers (below equation) to the oxygen transport capacity of a specific contact lens with thickness t, and it generally expressed in units of 10-9 cm mlO2/(s ml mmHg) or 10-9 mlO2/(s cm2 mmHg) [25].
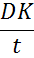
Seeking to improve soft contact lens permeability scientist started to make hydrogels from silicone based polymers like polydimethylsiloxane (PDMS). The silicone hydrogel contact lens, also known as siloxane lens, show impressive permeability (PDMS has a Dk of 600 barres), while retaining the comfort, wettability and biofilm resistance of non-silicon based hydrogels [26]. However, in order to avoid hypoxia, an extended wearable contact lens must provide at least minimum oxygen transmissibility (Dk/t) of 87 barrer, which may not be easy to attain with traditional hydrophilic contact lens [24]. Recently, one report suggested the minimum value of Dk/t to avoid hypoxia as 125 barrer [27]. The reported values of Dk of various commercial contact lenses are given in Table 1. With an approximate average thickness of 80 mm, these commercial siliconehydrogel contact lenses can provide sufficient oxygen transmissibility to be used as extend wear. Various researchers have used these extended wear contact lenses for ophthalmic drug delivery with no effect on the oxygen permeability of the lens [28-30].
Glass transition temperature
The Tg of contact lenses is not expected to alter during the drug loading process or due to incorporation of additives. Several studies indicate that the contact lenses manufactured by various approaches have not shown any significant effect on Tg [31]. Further, the alteration in Tg on addition of βCD was found to be insignificant, which suggests that the grafted βCD has little or no effect on the degree of cross-linking of the hydrogels or the stiffness of the network [32]. Costa et al also found that the Tg of contact lenses was not affected after the impregnation process and drug release [31]. Yanez et al reported no alteration in the Tg values of the SCLs with successive super critical fluid processing steps [33]. Thus, most of the methods used for the fabrication of contact lenses have good feasibility of loading the drugs with no change in Tg.
Wettability
Hydrophobic polymers will repel the water that makes up a majority of the tear surface. This disrupts the tear flow and results in the deposition of an albumin film on the lens, which eventually reduces the effectiveness of the contact and can cause infection and/or irritation [34]. Therefore, if a contact lens surface is highly hydrophobic it needs to be made hyhighdrophilic. Doping the polymer or treating the surface of the polymer can do this change in the morphology of the surface. However, wettability of soft contact lenses affects their physiological compatibility and the stability of the pre-lens lacrimal fluid and can be measured by contact angle measurements. For instance, wettability of pHEMA was slightly increased when copolymerized with high proportions of glycidyl methacrylate (GMA), but slightly decreased when βCD was attached [32]. However, contact lenses using β-CD-loading can be used for extended drug delivery. On the other hand, no significant effect on the contact angle was measured when timolol maleate and acetazolamide was impregnated using supercritical solvent impregnation technique [31]. The theoretical contact angles above 90º indicates non-wetting of the lens surface [35]. There are reports in the literature which suggests that supercritical fluid based contact lenses keep their wettability properties after processing [33]. In addition to wettability and content, the comfort of soft contact lens also depends on the viscoelasticity of the network and on its sliding over the eye surface and the lids [36]. All these results indicate that several processing techniques could be used for the loading of drugs in contact lenses without producing much effect on the wettability. Further one should be aware that contact lens should be highly hydrophilic and must resist the deposition of a biofilm on the lens, in the case of extended wear.
Water content
The permeability of the lens is proportional to the amount of water in the lens. As the percent weight of water increases in the lens, the permeability increases linearly. The ability of lenses to absorb large amounts of water also makes them highly hydrophilic. These attributes gives soft contact lenses the ability to achieve greater permeability and could be used for extended wear without disturbing the eye. However, achieving greater permeability is a complex issue as increase in water content will eventually loose the polymer strength. This can lead to tearing or scratching of the lens. A softer lens also offers the cornea less protection. Lenses with higher water content absorb more water soluble drug compounds and releases it later into the tear film than do low water content lenses. Nevertheless, there is no correlation exist between the amount of drug release and the water content of the lens for hydrophobic drug as shown by Kim et al [37]. The plausible reason is that the hydrophobic drugs will partition into the silicone rich phases, and the partition coefficient of drug in the gels will be influenced by the silicone composition of the gels.
Approaches For Drug Incorporation
Basically two approaches have been used to incorporate drugs into contact lenses; loading drugs into preformed lenses, or manufacturing the lens with the drugs entrapped inside. Attempts were also made by dissolving drug in the monomer solution and followed polymerization, incorporation of drug loaded nano or micro based vesicular systems into the matrix, ocular inserts, ion exchange reaction with hydrogel functional groups etc. However, these techniques increase the number of processing step and are tedious for fabrication. Various research groups have worked on novel approaches to enhance the drug delivery into the cornea using the application of contact lenses, and are summarized in Table 2.
| S. No. | Drugs used | Polymers/contact lens | Method of drug incorporation | Inference |
|---|---|---|---|---|
| 1. | Acetazolamide, timolol maleate | Balafilcon A | Discontinuous SSI methodology | Demonstrated the feasibility of preparing balafilcon A contact lenses using scCO2, ethanol and water by SSI [31]. |
| 2. | Acetazolamide or Ethoxzolamide | HEMA, zinc methacrylate, 1- or 4-vinylimidazole, and N-hydroxyethyl acrylamide | Bioinspired imprinted pHEMAhydrogels | Remarkable improvement in the performance as controlled release system [56]. |
| 3. | Azulene | HEMA + methacrylmide propyl trimethylammonium chroride | Molecular imprinting technique | Molecular imprinting is capable to store the anionic drug such as azulene based on ion-exchange reaction [57]. |
| 4. | Brimonidine tartrate | Acuvue contact lenses | Soaked in 0.1% brimonidine tartrate solution | Disposable contact lenses are able to uptake and release brimonidine tartrate in vitro [29]. |
| 5. | Ciprofloxacin, fluorescein | PLGA films over pHEMA | By ultraviolet light polymerization film coating process | PLGA film coated over pHEMA lenses sustained the release of drug, which can be controlled by changing either the ratio of drug to PLGA or the molecular mass of the PLGA used [50]. |
| 6. | Ciprofloxacin HCl | Lotrofilcon A, etrafilcon A, balafilcon A | Soaked in 0.3% ciprofloxacin-HCl | All materials released the drug too quickly to be effective as drug delivery device [41]. |
| 7. | Dexamethasone | Alphafilcon A, lotrafilcon A and galyfilcon | Soaked in 0.1% dexamethasone solution | None of the materials released drug for long period of time to be clinically useful as a drug delivery device [18]. |
| 8. | Dexamethsone | ACUVUE, OASYS, NIGHT & DAY and O2OPTIX | Soaking of vitamin E loaded lens in drug solution | Vitamin E loading increases the drug release by 9 to 16 fold than non vitamin E loaded lenses [37]. |
| 9. | Diclofenac sodium | pHEMA and GMA | β-CD was grafted to the gel network and soaked in drug solution. | The hydrogels with pendant β-CD are particularly useful for the development of cytocompatible drug loaded SCLs [32]. |
| 10. | Flurbiprofen | Hilafilcon B | Imprinting of contact lens by supercritical fluid-assisted method | Shorter process time than those using conventional aqueous-based molecular imprinting methods [33]. |
| 11. | Flurbiprofen, timolol maleate | Nelfilcon A, omafilcon A methafilcon A and hilafilcon B. | Discontinuous SSI methodology | SSI can be considered as a viable, safe, and efficient method for impregnation of drugs [19]. |
| 12. | Gentamicin | HEMA with 38.6% water content | Soaking in 0.5% drug solution | Bactericidal concentrations were found up to 3 days after contact lens fitting in all human subjects [38]. |
| 13. | Gentamicin | Morgan therapeutic lens (MTL) | Soaking in 1 mg/ml and 5 mg/ml solutions | Gentamicin concentrations attained via the MTL exceeded the mean inhibitory concentration for most sensitive bacterial species in rabbit’s cornea [58]. |
| 14. | Gentamicin, Kanamycin, Tobramycin Ciprofloxacin, Ofloxacin | Acuvue contact lens | Soaking of lenses in 0.3% drug solutions | It releases sufficient amounts of gentamicin, ciprofloxacin and ofloxacin to produce bacteriostatic concentrations in the aqueous humor in cataract patients [39]. |
| 15. | Hexadecane | pHEMA | Dispersion of hexadecane microimuslsion in contact lenses | p-HEMA gels loaded with a microemulsion, stabilized with silica shell are transparent and releases drugs for a period of over 8 days in vitro [22]. |
| 16. | Hyaluronic acid (HA) | Nalfilcon A | Molecular imprinting technique | HA can be delivered from a disposable lens at a therapeutic rate of approximately 6 μg/h for 24 h [45]. |
| 17. | Ketotifen, diclofenac sodium | HEMA, acrylic acid, acrylamide and methacrylic acid | Gels prepared by living /controlled imprinting technique | Imprinting via living polymerization extends or delays the template release profile by two-fold over that of imprinting via conventional free-radical polymerization techniques [43]. |
| 18. | Ketotifen fumarate | Bitelechelic methacrylated polydimethylsiloxanes macromonomer, 3-methacryloxypropyltris (trimethylsiloxy)silane, and N,Ndimethylacrylamide using ethylene glycol dimethacrylate | Pre-soaking in drug solution | Sustained ketotifen fumarate was released for more than 24 h in rabbit’s eye [59]. |
| 19. | Lomefloxacin | Acuvue contact lens | Soaked in commercially-available 0.3% lomefloxacin eye solution | Releases sufficient amounts of lomefloxacin for up to 8 h (Rabbit’s eye) [28]. |
| 20. | Norfloxacin | pHEMA with acrylic acid | Mollecular imprinting technique | Imprinted hydrogels synthesized using norfloxacin: acrylic acid (1:3 and 1:4) molar ratios showed the greatest ability to control the release process, sustaining it for more than 24 h [60]. |
| 21. | Puerarin | pHEMA, HEMA, mono-methacrylated β-CD (mono-MA-β-CD) and trimethylolpropane trimethacrylate | Prepared by photopolymerization | The data demonstrate that pHEMA/β-CD hydrogel contact lenses can effectively deliver puerarin through the rabbit’s cornea [61]. |
| 22. | Timolol | HEMA+ methacrylic acid or methmethacrylate | Molecular imprinting technique | Incorporation of methmethacrylate as comonomer increases the timolol loading capacity to therapeutically useful levels while retaining appropriate release characteristics in vitro [62]. |
| 23. | Timolol | N-N-diethyllacrylamide, methacrylic acid, ethylene glycol dimethacrylate | Molecularly imprinted technique | Timolol loading capacity of the contact lenses as well as sustaining of drug release was improved by the molecular imprinting method [63]. |
| 24. | Timolol, dexamethasone, dexamethasone- 21-acetate | N,N-dimethylacrylamide, 3-me thacryloxypropyltris(trimethyls iloxy)silane, bis-alpha, omega- (methacryloxypropyl) polydimethylsiloxane, 1-vinyl-2-pyrrolidone, and ethylene glycol dimethacrylate | Soaked in different drug solutions concentrations | The mechanical and physical properties of lenses of the hydrogels are suitable for contact lens application [49]. |
| 25. | Timolol, fluconazole, dexamethason-21- diphosphate | NIGHT&DAYTM lens | Soaking of vitamin E loaded lens in drug solution | Increased the release time of drugs but vitamin E loading have slight effect on the mechanical property of lens [25]. |
Table 1: Various approaches and drugs used for contact lens drug delivery.
Drug loading in preformed lenses
The success of ocular delivery using contact lenses is based on its ability to release the drug at controlled rate for an extended period of time. Traditionally, contact lens are applied by soaking it on a drug solution for a short duration and placed over the eye surface. However, the therapeutic efficiency of the commercial contact lens was not very successful and the treatment remains elusive. The major cause for this is due to the very low uptake of drug by the lens and the rapid release which eventually leads to low residence time. Key limitations of soaking methods are the diffusion of water into the polymer and drug aqueous solubility. At present, the modern manufacturing techniques could be utilized to formulate extended wear contact lens (using silicone hydrogel) which are permeable to oxygen and can be worn for long duration. Using this as an advantage several contact lenses are fabricated and evaluated [28,38]. Efficiency of acuvue lenses was assessed after loaded with lomefloxacin, by immersing it in commercially available eye drops and placing on the cornea of albino rabbits. The drug concentration in the corneal and aqueous humor levels of lomefloxacin in the eyes following the wear of lomefloxacin-loaded lenses were found to be significantly higher than those achieved by frequent-drop therapy [28]. Hehl et al reported that acuvue contact lenses could be the most feasible drug delivery system for fluoroquinolones [39]. Further, Karlgard et al measured the uptake and release of several drugs using commercially available HEMA based and extended-wear silicone contact lenses (ACUVUE®, OASYS™, NIGHT & DAY™ and O2OPTIX™), which showed rapid release (~10 min) of drug suggesting its failure as an extended drug delivery [40]. Similar results were observed by other researchers when the study was carried out using ciprofloxacin and dexamethsone [18,41]. In another attempt, Schultz and Morck [30] have explored the feasibility of drug uptake and release using epidermal growth factor from different hydrogel contact lenses in rabbit model. The results indicate that epidermal growth factor can be delivered from some but not all hydrogel materials, suggesting that silicon polymers are specific to drugs.
Newer Approaches
The use of polymers with varying width of channels in the matrix can control drug delivery rate which remains effective for longer periods. In this contest, soft contact lenses are more preferred due to their potential of increasing bioavailability of ophthalmic drugs [42]. Approaches like molecular imprinting, particle-laden soft contact lenses, barrier approach, complexation have been proposed to improve the corneal drug delivery. The pros and cons of various approaches are discussed below;
Molecular imprinting
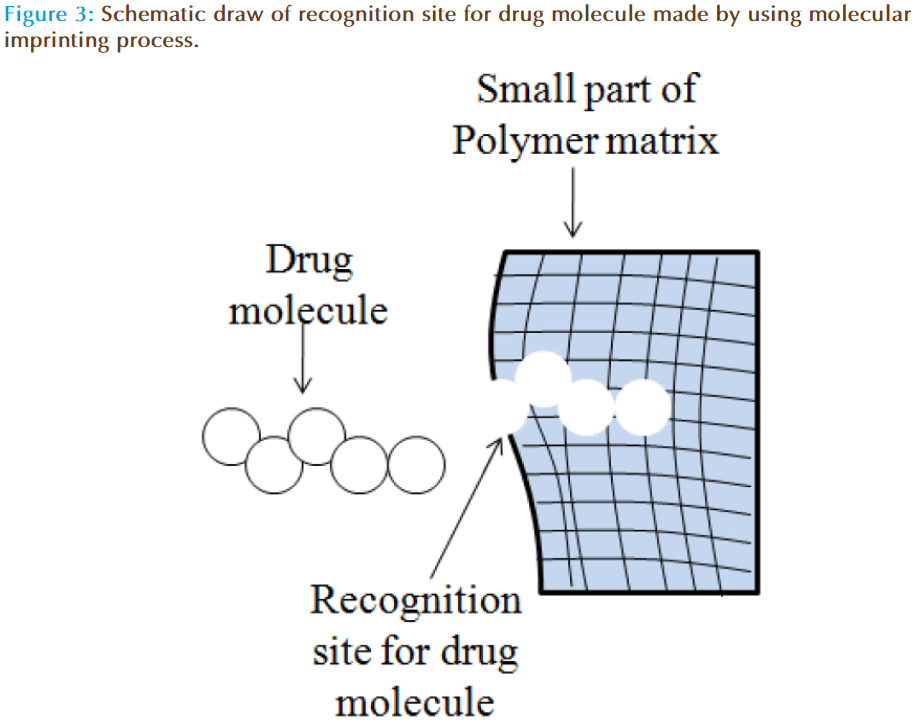
The concept of molecular imprinting within the gels involves creation of macromolecular memory for a template molecule within a polymer network. The diffusion of drug molecules from imprinted contact lenses can be influenced by average mesh size, template size and drug-polymer chain interactions [43]. Manipulation of these variables can increase the partition coefficient and slow down the release of drug. Indeed, zero-order or concentration independent release kinetics is highly desirable from drug delivery devices. This can be achieved by controlling the therapeutic loading, providing careful attention to the functional monomer, template ratio, the diversity of functional monomers, the polymer backbone and network structure. Ali et al have demonstrated the potential of molecular imprinting to tailor therapeutic release kinetics of ketotifen fumarate (MW=425) by imprinting process [44].
Molecular imprinting is a polymer synthesis technique exploiting template-medicated polymerization mechanisms to produce synthetic macromolecular networks with tailored affinity, capacity and selectivity for a template molecule which is depicted schematically in Figure 3. This approach provides a rational design strategy for the development of therapeutic contact lenses with enhanced loading and delayed release. Using these strategies, hydrogel contact lenses were designed which are capable of releasing hyaluronic acid at a controlled rate (~6 μg/h for 24 h) [45]. Progress in the field has leads to development of ODDS of low-molecular-weight therapeutics such as anti-glaucoma, antihistamines, antibiotics, anti-inflammatories etc for the treatment of anterior eye disorders. Experimental work has also confirmed that macromolecular memory and not structural differences are highly responsible for controlling drug release kinetics, compared with non-imprinted systems [46]. Further, it is also found that the therapeutically relevant amount of drug can be loaded and released over an extended period of time, which allows the technique to be applied to daily-wear and extended-wear contact lenses.
Particle-laden soft contact lenses
Particle-laden hydrogels are promising approach for ocular drug delivery and are expected to deliver the drugs at therapeutic levels for a period of time. It involves liposomes-laden, surfactant laden, biomimetic hydrogels and drug polymer films coated with hydrogels. It may be possible to use this system for both therapeutic drug delivery to eyes and the provision of lubricants to alleviate eye problems prevalent in extended lens wear [23]. Typically, they are transparent and provide controlled drug delivery. Gulsen and Chauhan encapsulated the ophthalmic drug formulations in nanoparticles and dispersed these drug-laden particles in the lens material, such as p-HEMA hydrogels. This drug-laden p-HEMA hydrogels were synthesized by free radical solution polymerization of the monomers in presence of nanoparticles and were found to control the drug release for few days [47]. Similarly, to reduce drug loss and side effects, it is proposed to encapsulate the ophthalmic drug formulations in liposomes and disperse the drug-laden liposomes in the lens material. Upon insertion into eye, the liposome-laden lens is likely to release the drug between air and lens and/or between cornea and lens, thus provides drug delivery for extended periods of time. In another study, dimyristoyl phosphatidylcholine liposomes are dispersed in p-HEMA hydrogel contact lenses and the results indicate the prepared lenses are transparent and exhibited controlled release (~8 days). Further, the delivery rates can be modified by controlling particle size and drug loading [23]. Alternatively, drug delivery from contact lenses can be controlled by formulating microencapsulation. Gulsen et al embedded microemulsion drops in p-HEMA hydrogels, stabilized with a silica shell, which were found to be transparent and provided controlled release for >8 days. Kapoor and Chuhan have developed surfactant-laden p-HEMA contact lenses of cyclosporine A for the controlled release using various Brij surfactants and evaluated the influence of chain length and unsaturated groups on drug release. The results indicate that surfactant-laden p-HEMA gels are potential for extended release of cyclosporine A, and possess suitable mechanical and optical properties for contact lens applications [48]. All these studies points to the fact that particle-laden hydrogels could be considered as one of the most promising approaches for the successful delivery of ODDS.
Barrier approach
Another approach for the controlled release of drug moieties from contact lenses is by in situ creation of transport barriers. This diffusion barrier can be any solid or liquid material that is impermeable to ophthalmic drugs. However, the prerequisite is that the barrier could be dispersed within the lens material in a manner that keeps the lens transparent. Several ophthalmic drugs are charged at physiological pH and so hydrophobic molecules will likely form effective barriers. It is also important to ensure that the barrier material is biocompatible so that diffusion of the compound from the barrier into the tear film does not cause toxicity. Kim et al developed extended wear silicone hydrogel soft contact lenses that deliver ophthalmic drugs for an extended period of time ranging from weeks to months. Contact lenses comprising of N, N-dimethylacrylamide, 3-methacryloxypropyltris (trimethylsiloxy) silane, bis-alpha, omega-(methacryloxypropyl) polyethyl, 1-vinyl-2 -pyrrolidone, and ethylene glycol dimethacrylate were formulated using varying ratios of monomers and evaluated for the drug diffusion of timolol, dexamethasone, and dexamethasone 21-acetate from the silicone hydrogels. The results from these studies indicate that these polymers provides diffusion limited transport [49].
Prototype, drug-eluting contact lenses were designed by coating poly(lactic-co-glycolic acid) (PLGA) films containing fluorescein or ciprofloxacin with pHEMA by ultraviolet light polymerization. The results demonstrated controlled release of the molecules with zero-order release kinetics for over 4 weeks. Thus, it is beneficial to use drug-PLGA film coated with pHEMA as a platform for controlled and sustained release with widespread therapeutic applications in ocular drug delivery [50]. Vitamin-E which is a hydrophobic liquid has potential benefits as biocompatibility and therapeutics, is recently used as the diffusion barrier. Drugs with different physicochemical properties such as timolol, dexamethasone 21-disodium phosphate and fluconazole were widely investigated. The data demonstrated that loading of 10 and 40% vitamin E increases the release time of timolol by a factor of about 5 and 400, respectively. Similar results have been obtained for other hydrophilic drugs including fluconazole and dexamethasone 21-disodium phosphate [25]. However, vitamin E uptake may increase the size of the contact lenses but there is ~8% increase in wet diameter when ~40% of vitamin E is incorporated, which is likely to be tolerated by human eyes. On the other hand, incorporation of vitamin E has lead to extend the drug release to 7 to 9 days, which is 9 to 16 fold more as compared to the dexamethasone release from pure contact lens, devoid of vitamin E [37]. All these observations give initiative for the use of vitamin E loaded contact lenses for controlled and extended delivery of both hydrophobic and hydrophilic drugs.
Cyclodextrin for controlled drug delivery
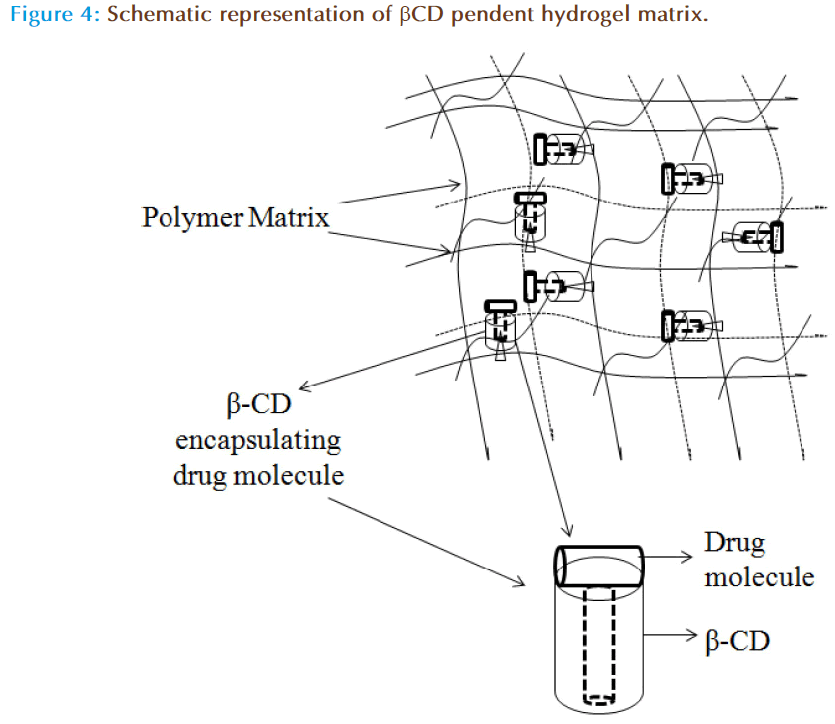
Cyclodextrins (CDs) are cyclic oligosaccharides with hydrophobic cavity and form inclusion complex with several drug moieties through reversible non-covalent interaction, and have shown success in ODDS as well. The activity of CDs in liquid formulations is limited as the decomplexation is instantaneous; however, in a polymeric network the release of the drug is controlled. By forming the inclusion complexes they may load a drug efficiently and their release could be sustained for several days. In addition, pHEMA hydrogels were prepared by copolymerization with glycidyl methacrylate at various proportions and then beta-cyclodextrin (β-CD) was grafted to the network by reaction with the glycidyl groups under mild conditions. This led to networks in which the β-CDs form no part of the structural chains but they are hanging on 2-3 ether bonds through the hydroxyl groups. The schematic representation of the β-CD loaded polymer matrix is shown in the Figure 4. However, the data observed suggests that grafted β-CD does not alter the Tg, swelling, optical transparency, or oxygen permeability of the network, but enhanced the drug loading (~1300%) and drug affinity (~15 fold). These contact lenses showed extended release for 2 weeks in lacrimal fluid with no leakage in the conservation liquid of contact lenses [32]. Xu et al have prepared pHEMA/β-CD hydrogel membranes and contact lenses by photopolymerization of HEMA, mono-methacrylated β-CD (mono-MA- β-CD) and trimethylolpropane trimethacrylate by cast molding process and assessed the release pattern of puerarin (a chinese extract to alleviate glaucoma and ocular hypertension). The data suggests that the drug loading and in vitro release rate were dependent on β-CD content in the pHEMA/β-CD hydrogels and exhibits greater bioavailability, long residence time in tear fluid of rabbit eyes when compared to pHEMA contact lenses and eye drops [51]. These results shows that the hydrogels with pendant cyclodextrins are particularly useful for the development of cytocompatible medicated implants or biomedical devices, such as drug-loaded soft contact lenses as they does not significantly alter the Tg, swelling, optical transparency or oxygen permeability of the network with increase in drug load.
Supercritical solvent impregnation
Drugs may also be impregnated and dispersed into polymeric matrixes by dissolving them in compressed high volatile fluids (like carbon dioxide) at temperatures and pressures near or above their critical temperatures and pressures, and contacting the resulting mixtures with those polymeric matrixes. This may removes the limitations of conventional methods, like the possible use of toxic organic chemicals, drug/solvents dissolution and compatibility issues, undesired drug reactions, drug photochemical and thermal degradation, low incorporation yields and heterogeneous drug incorporation/dispersion [52,53]. However, the compressed fluid mixture facilitates the diffusion of the drugs into the polymer matrix and act as a swelling agent or plasticizer for polymers, thus increasing the free volume. In addition, utilization of cosolvents can augment the overall process by increasing the drug solubility or by increasing the swelling and plasticization. On the whole, the amount of drug incorporation can be controlled by altering the operational pressure, processing temperature and selecting an apposite solvent.
The supercritical solvent impregnation (SSI) technique has already proved its significance in the development of drug impregnated polymeric materials, which may have applications in drug delivery through therapeutic contact lenses. SSI allows the drug impregnation/dispersion of most polymeric articles and, when properly employed, without altering and/or damaging their physical, chemical, and mechanical properties and without degrading their constituent drugs, additives and polymers. Further, SSI proved to be a tunable process since the variation of the employed operational conditions indicated that it is possible to control the amount of impregnated drug [19]. Moreover, this technique provides the opportunity to incorporate both hydrophilic and hydrophobic drugs into the contact lens. Two different methods of super critical carbon dioxide (scCO2) impregnation/deposition of drugs were explained as
a) Supercritical fluid deposition or dispersion: In this scCO2–drug mixture will not really dissolve in the polymeric material but will diffuse into the existing pores of the material. On venting, the drug will nucleate, grow and precipitate as solid particles inside the pores but only on the surfaces of the polymeric material and may not be molecularly dispersed inside the polymer.
b) Supercritical fluid impregnation (or supercritical solvent impregnation): - In this scCO2– drug mixture will dissolve in the polymeric material, promoting its inner plasticization and/ or swelling. On venting, the drug will nucleate, grow and precipitate both inside the pores (at their surfaces, not molecularly dispersed) and inside the previously swollen polymeric material (molecularly dispersed) [19].
Recently, Costa et al evaluated the effects of nature and concentration of cosolvents (ethanol and water) on the impregnation efficiency and the properties of the contact lenses [31]. The results showed that final impregnation yield increases with the incorporation of co-solvent due to the increase in solubility of the drug with no effect on the oxygen permeability, wettability and the Tg of contact lenses. Thus, SSI can be consider as a viable, efficient and safe alternative for the impregnation of drugs, including those of hydrophobic character or presenting low aqueous solubility, into commercially available soft contact lenses by using cosolvents.
Clinical Investigations
Several studies in human have been conducted to evaluate the novel contact lenses. In one attempt, Busin and Spitznas have assessed the activity of hydrogel bandage contact lenses (61% HEMA and 38.6% water content) soaked overnight in commercially available sterile gentamicin solution (0.5%), and placed on eyes of healthy adult volunteers. The results indicated greater (>minimum inhibitory concentration) and extended release (~3 days) of drug in the corneal region [38]. In an another study, 466 patients having cataract were given chloromycetin, gentamicin, or carbenicilli subconjunctivally and using New Sauflon 70 and New Sauflon 85 lenses. The results observed indicated significantly higher penetration of drugs by soft contact lenses than subconjunctival therapy for a period of ~12 h [54]. Recent study by CIBA VISION® using novel DAILIES® AquaComfort Plus® contact lenses indicates significant reduction in common contact lens related symptoms. Bausch and Lomb Clinical Research conducted 27 controlled extended wear clinical trials using PureVision lenses manufactured from a highly oxygen permeable, low water content (36%), surface treated silicone hydrogel material, balafilcon A for the presence of microbial keratitis. In Boston, a prototype two layers contact lens has been designed using PLGA and pHEMA has been found to release the drug for more than 50 days, when tested in humans. Studies on the long term effects of the extended wear of senofilcon A silicone hydrogel contact lenses on ocular tissues has reported excellent biocompatibility and ocular tissue tolerance [55]. Moreover, several studies in humans are undergoing at various research centers to ensure the efficiency, safety, biocompatability and better patient compliance of the newly fabricated contact lenses.
Conclusion
Progress in the field of contact lenses drug delivery has been established recently with controlled loading and sustained release. Different techniques have been used for increasing the drug load and controlled release. Each technique may have some pros and cones with little effect on mechanical and optical properties of the lens. Various lens materials and their requirement for ophthalmic use, also have effect on the drug loading. Experimental works demonstrated that the contact lenses exhibit greater drug loading with adequate mechanical and optical properties. The type of the contact lenses and the technique of drug loading are found to affect the residence time of the drug. In comparison with topical alternatives, contact lenses provide an increased residence time at the surface of the eye for efficacious therapy.
Conflict of Interest
There are no conflicts of interest.
References
- Clark AF, Yorio T. Ophthalmic drug discovery. Nat Rev Drug Discov. 2003; 2(6): 448-59.
- Yorio T, Wilson SE, Clark AF, Wax MB. Refractive surgery - Corneal opacity (haze) after surface ablation. In: Ocular therapeutics: eye on new discoveries. Wax MB, Clark AF, Yorio T, eds. Amsterdam, MA: Academic; 2008. p. 133-41.
- Amo E, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008; 13(3-4): 135–43.
- Ding S. Recent developments in ophthalmic drug delivery. Pharm Sci Technol Today. 1998; 1(8): 328–35.
- Urtti A. Challenges and obstacles of ocular pharmacokinetics. Adv Drug Deliv Rev. 2006; 58(11): 1131–5.
- Bourlais C, Acar L, Zia H, et al. Ophthalmic drug delivery systemsrecent advances. Prog Retin Eye Res. 1998; 17(1): 33–58.
- Burrows J, Tsibouklis J, Smart JD. Drug delivery to the eye, technical report. In: The drug delivery companies report 2002, Pharmaventures Ltd., Oxford, UK, 2002.
- Costa VP, Harris A, Stefansson E, et al. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog Retin Eye Res. 2003; 22(6): 769–805.
- Sasaki H, Yamamura K, Nishida K, et al. Delivery of drugs to the eye by topical application. Prog Retin Eye Res. 1996; 15: 583–619.
- Merkli A, Tabatabay C, Gurny R, et al. Biodegradable polymers for the controlled release of ocular drugs. Prog Polym Sci. 1998; 23(3): 563–80.
- Lloyd AW, Faragher RGA, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001; 22(8): 769–85.
- Reddy IK, Ganesan MG. Ocular therapeutics and drug delivery: an overview. In: Ocular therapeutics and drug delivery: A multidisciplinary approach. Reddy IK, eds. Philadelphia (USA): Technomic publishing company Inc; 1996. p. 3–29.
- Baeyens V, Gurny R. Chemical and physical parameters of tears relevant for the design of ocular drug delivery formulations. Pharm Acta Helv. 1997; 72(4): 191–202.
- Urtti A, Salminen L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv Ophthalmol. 1993; 37(6): 435–56.
- Shell JW. Pharmacokinetics of topically applied ophthalmic drugs. Surv Ophthalmol. 1982; 26: 207–18.
- Sedlacek J. Possibility of application of eye drugs with the aid of gel-contact lenses, Cesk Oftalmol. 1965; 21: 509–12.
- Lang JC. Ocular drug delivery: conventional ocular formulations. Adv Drug Deliv Rev. 1995; 16(1): 39-45.
- Boone A, Hui A, Jones L. Uptake and release of dexamethasone phosphate from silicone hydrogel and group I, II, and IV hydrogel contact lenses. Eye Contact Lens. 2009; 35(5): 260-7.
- Costa VP, Mara EM, Braga A, et al. Development of therapeutic contact lenses using a supercritical solvent impregnation method. J Supercrit Fluids. 2010; 52: 306–16.
- Bennett ES. Material Selection. In: Clinical Manual of Contact Lenses. Bennett ES, Henry VAJB eds. Philidelphia (USA): Lippincott Company; 1994. p. 27-40.
- Chou B. The evolution of silicone hydrogel lenses. Contact lenses spectrum. 2008; 22(6): 37-39.
- Gulsen D, Chauhan A. Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. Int J Pharm. 2005; 292(1-2): 95-117.
- Gulsen D, Li CC, Chauhan A. Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery. Curr Eye Res. 2005; 30(12): 1071-80.
- Holden B, Mertz G. Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses. Invest Ophthalmol Vis Sci. 1984; 25: 1161–7.
- Peng CC, Kim J, Chauhan A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing vitamin E diffusion barriers. Biomaterials. 2010; 31(14): 4032-47.
- Friends GD, Lai YC. Surface wettability enhancement of silicone hydrogel lenses by processing with polar plastic molds. J Biomed Mater Res. 1997; 35: 349-56.
- Harvitt D, Bonanno J. Re-evaluation of the oxygen diffusion model for predicting minimum contact lens Dk/t values needed to avoid corneal anoxia. Optom Vis Sci. 1999; 76(10): 712–9.
- Tian X, Iwatsu M, Kanai A. Disposable 1-day Acuvue contact lenses for the delivery of lomefloxacin to rabbits eyes. CLAO J. 2001; 27(4): 212-5.
- García DS, García Gómez S, Barreiro Rego A, et al. Brimonidine absorption and release from one day acuvue disposable contact lenses. Arch Soc Esp Oftalmol. 2001; 76(10): 599-603.
- Schultz CL, Morck DW. Contact lenses as a drug delivery device for epidermal growth factor in the treatment of ocular wounds. Clin Exp Optom. 2010; 93(2): 61-5.
- Costa VP, Mara EM, Braga A, et al. Anti-glaucoma drug-loaded contact lenses prepared using supercritical solvent impregnation. J Supercrit Fluid. 2010; 53:165–73.
- Santos DJF, Alvarez-Lorenzo C, Silva M, et al. A soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterial. 2009; 30(6): 1348-55.
- Yañez FA, Martikainen LB, Mara EM, et al. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomaterialia. 2010; 6: 3919-26.
- Rao JB, Saini JS. Complications of contact lenses. In: Contact Lenses. Aquavella JV, Rao GN, Philidelphia (USA): Lippincott Company; 1987. p. 195-225.
- Compan V, Andrio A, López-Alemany E, et al. Oxygen permeability of hydrogel contact lenses with organosilicon moieties. Biomaterials. 2002; 23(13): 2767–72.
- Refojo MF, Leong FL. Poly(methylacrylate-co-hydroxyethylacrylate) hydrogel implant material of strength and softness. J BiomedMater Res. 1981; 15: 497–509.
- Kim J, Peng CC, Chauhan A. Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. J Control Release. 2010; 148(1): 110-6.
- Busin M, Spitnas M. Sustained gentamicin release by presoaked medicated bandage contact lenses. Ophthalmology. 1988; 72: 150–4.
- Hehl EM, Beck R, Luthard K, et al. Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses. Eur J Clin Pharmacol. 1999; 55(4): 317-23.
- Karlgard C, Wong NS, Jones L, et al. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int J Pharm. 2003; 257(1-2): 141–51.
- Hui A, Boone A, Jones L. Uptake and release of ciprofloxacin-HCl from conventional and silicone hydrogel contact lens materials. Eye Contact Lens. 2008; 34(5): 266-71.
- Xinming L, Yingde C, Lloyd AW, et al. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: a review. Cont Lens Anterior Eye. 2008; 31(2): 57-64.
- Ali M, Vaughan AD, Zhang J, et al. Templated hydrogels for combination devices. In: Therapeutic contact lenses. Ali M, Vaughan AD, Zhang J, eds. Proceedings of the 31st Annual international conference of the EEE engineering in medicine and biology society; 2009 Sept. 3-6; Michigan, United States: Wichtig editore-medical publisher; 2009. p. 242-5.
- Ali M, Horikawa B, Venkatesh SA, et al. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J Control Release. 2007; 124(3): 154–62.
- Ali M, Byrne ME. Controlled release of high molecular weight hyaluronic acid from molecularly imprinted hydrogel contact lenses. Pharm Res. 2009; 26(3): 714-26.
- White CJ, Byrne ME. Molecularly imprinted therapeutic contact lenses. Expert Opin Drug Deliv. 2010; 7: 765-80.
- Gulsen D, Chauhan A. Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci. 2004; 45: 2342-7.
- Kapoor Y, Chauhan A. Drug and surfactant transport in cyclosporine A and brij 98 laden p-HEMA hydrogels. J Colloid Interface Sci. 2008; 322(2): 624-33.
- Kim J, Conway A, Chauhan A. Extended delivery of ophthalmic drugs by silicone hydrogel contact lenses. Biomaterials 2008; 29(14): 2259-69.
- Ciolino JB, Dohlman CH, Kohane DS. Contact lenses for drug delivery. Semin Ophthalmol. 2009; 24(3): 156-60.
- Xu J, Li X, Sun F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomaterialia. 2010; 6: 486–93.
- Duarte ARC, Coimbra P, De Sousa HC, et al. Solubility of flurbiprofen in supercritical carbon dioxide. J Chem Eng Data. 2004; 49: 452–99.
- Coimbra P, Duarte ARC, De Sousa HC, et al. Solubility of flurbiprofen and timolol maleate in dense carbon dioxide. Brunner G, Kikic I, Perrut M, eds. Proceedings of the 6th International Symposium on Supercritical Fluids (ISASF); 2003 28-30th April; Versailles, France: 2003. p. 775–80.
- Jain MR. Drug delivery through contact lenses. Br J Ophthalmol. 1988; 72: 150–4.
- Guillon M, Maïssa C. Long-term effects of the extended wear of senofilcon A silicone hydrogel contact lenses on ocular tissues. Optometry. 2010; 81(12):671–9.
- Ribeiro A, Veiga F, Santos D, et al. Imprinted pHEMA-Hydrogels for ocular delivery of carbonic anhydrase inhibitor drugs. Biomacromolecules. 2011; 12(3): 701–9.
- Uchida R, Sato T, Tanigawa H, et al. Azulene incorporation and release by hydrogel containing methacrylamide propyltrimenthylammonium chloride, and its application to soft contact lens. J Control Release. 2003; 92(3): 259-64.
- Rootman DS, Willoughby RP, Bindlish R, et al. Continuous flow contact lens delivery of gentamicin to rabbit cornea and aqueous humor. J Ocul Pharmacol. 1992; 8: 317-23.
- Xu J, Li X, Sun F. In vitro and in vivo evaluation of ketotifen fumarateloaded silicone hydrogel contact lenses for ocular drug delivery. Drug Deliv. 2011; 18(1):150-8.
- Alvarez-Lorenzo C, Yañez F, Barreiro-Iglesias R, et al. Imprinted soft contact lenses as norfloxacin delivery systems. J Control Release. 2006; 113(3): 236-44.
- Xu J, Li X, Fuqian S. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomaterialia. 2010; 6: 486–93.
- Alvarez-Lorenzo C, Hiratani H, Concheiro A. Contact lenses for drug delivery, Achieving sustained release with novel systems. Am J Drug Deliv. 2006; 4(3): 131–51.
- Hiratani H, Fujiwara A, Tamiya Y, et al. Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials. 2005; 26 (11): 1293–8.