Interstitial PDT on Malignant Gliomas: About the Possible Relation between Optical Spectral Online Monitoring and T1 Hyper intensity in MRI
2 Department of Urology, University Hospital, LMU Munich, 81377 Munich, Germany
3 Department of Neurosurgery, University Hospital LMU Munich, 81377 Munich, Germany
Received: 15-Dec-2022, Manuscript No. Jbclinphar-22-83586; Editor assigned: 19-Dec-2022, Pre QC No. Jbclinphar-22-83586; Reviewed: 26-Dec-2022 QC No. Jbclinphar-22-83586; Revised: 09-Jan-2023, Manuscript No. Jbclinphar-22-83586 (R); Published: 16-Jan-2023
Citation: Aumiller M, Quach S, Rühm A, et al. Interstitial PDT on Malignant Gliomas: About the Possible Relation between Optical Spectral Online Monitoring and T1 Hyper intensity in MRI. J Basic Clin Pharma.2023;14(1):216-219.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Interstitial Photodynamic Therapy (iPDT) for malignant gliomas using 5-ALA and cylindrical light-diffusing fiber applicators is currently under development. In a preceding investigation, the effects of 5-ALA iPDT on haemoglobin-related light absorption in a liquid phantom model with intact erythrocytes and protoporphyrin IX were experimentally assessed. Spectral monitoring identified a light-induced formation of deoxygenated haemoglobin and methaemoglobin after the onset of iPDT irradiation.
This study summarises the retrospectively analysed spectral data acquired during clinical iPDT interventions of malignant gliomas. The results were analysed for correlations with early postoperative T1-weighted MRI data obtained without a contrast agent. Intrinsic T1-hyperintensity signals were observed and found to be associated with the treatment volume in all patients. This may indicate the presence of methaemoglobin, possibly induced by iPDT. From spectrally resolved recordings of light detected through each of the inserted cylindrical diffusers during tissue illumination through one of the other diffusers, treatment light transmission through and PpIX fluorescence of the tissue could be evaluated. The optical absorption coefficient μa and its change due to iPDT irradiation were estimated for all tissue volumes between evaluable fiber pairs. In cases with intrinsic T1-hyperintensity involvement, the absorption increase during iPDT-irradiation was significantly higher than in cases with minor T1-hyperintensity (p=0.003).
The observations are consistent with in-vitro experiments and indicate iPDT-induced deoxygenation of haemoglobin and formation of methaemoglobin, potentially after injury to capillary vessels. Further clinical investigations are needed to provide more data on the time course of the observed changes, thus paving the way for optimized irradiation protocols.
Keywords
5-aminolevulinic acid, protoporphyrin IX, photodynamic therapy, interstitial photodynamic therapy, malignant glioma, spectral online monitoring, haemoglobin, methaemoglobin, MRI, T1-hyperintensity
Introduction
Malignant gliomas are primary brain tumors with dismal outcomes [1,2]. Standard therapy consisting of fluorescence-guided resection followed by radio chemotherapy leads to a median survival of 15 months. Unfortunately, resection is limited if the tumour is localized in eloquent brain regions [3]. For these patients, alternative therapies are needed. 5-Aminolevulinic acid (5-ALA) mediated Interstitial Photodynamic Therapy (iPDT) is a treatment approach using 5-ALA as a prodrug, whose mechanisms are well explored [4]. It is orally administered and results in the selectively metabolized Protoporphyrin IX (PpIX), which acts as a photosensitizer in the tumor volume. Via irradiation of the tissue volume through Cylindrical Diffuser Fibers (CDF), which are stereotactically inserted into the tumour, the PpIX is activated to induce intracellular O2 and Reactive Oxygen Species (ROS), resulting in phototoxic reactions in the tumour cells (iPDT-irradiation parameters: 200 mW/(cm active CDF length) at 635 nm for 60 min resulting in 720 J/(cm active CDF length)) [5-7].
Further investigations on 5-ALA mediated iPDT focus on light delivery and treatment planning concepts for which optical tissue properties are essential [8]. Blood is the brain’s dominant absorber among the substances defining the optical properties of brain tissue (water, melanin and lipofuscin) [9-11]. The optical absorption of blood depends on the haemoglobin’s oxygenation and oxidation status. In-vitro studies on the impact of iPDT-irradiation on haemoglobin showed that during PpIXbased iPDT, the quantity ratio between the haemoglobin species changes over irradiation time, inducing a change in optical tissue properties [12]. Monitoring modalities can give insight into such effects during iPDT-irradiation in terms of optical monitoring techniques or could be assessed by clinical MRI or CT after irradiation.
In this study, Spectral Online Monitoring (SOM) data acquired during clinical iPDT on malignant gliomas were analysed concerning the measured signal changes [5]. Next, MRI data were analysed targeting intrinsic T1-hyperintensity visible in the native (non-contrast enhanced) T1-weighted MRI acquired the day after iPDT. Both observations were compared to find correlations, as changes in the SOM signals and intrinsic T1-hyperintensity are signs of inflowing blood during therapy. The ethical approval for the retrospective analysis was given by the institutional review board of the Faculty of Medicine at LMU Munich.
Literature Review
T1-hyperintensity in MRI
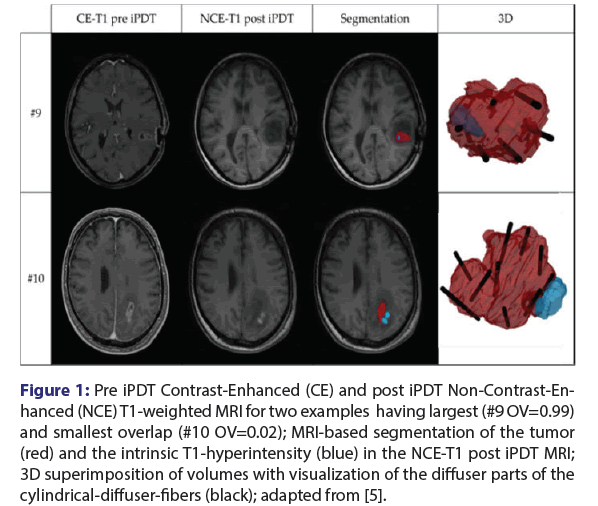
According to the iPDT-treatment protocol, MRI is acquired in the week before iPDT for treatment planning, and a post-therapeutic MRI is conducted on the day after iPDT (median 22 h). MRI assessment focused on treated tumor volume and an intrinsic T1-hyperintensity, which was newly discernible in early MRI imaging after irradiation [5,13]. A semi-automatic volumetric segmentation of the volumes of interest was performed on T1-weighted MRI with Gadolinium-based Contrast Enhancement (CE) and without (NCE), as shown exemplarily for two cases in Figure 1. The tumor volume was defined based on the CE-T1 MRI before (pre) iPDT. Based on NCE T1-MRI pre- and posttherapy, the newly detected intrinsic T1-hyperintensity was identified. Only if the T1-hyperintensity was apparent in the post-image but not the pre-image was it classified as iPDT-related. A T1-hyperintensity signal usually occurs due to the oxidation of haemoglobin to methaemoglobin and the change in the iron ionisation in the molecule [14]. The visibility of methaemoglobin in the MRI after therapy or surgery is a sign of bleeding or haemorrhages in the brain related to the intervention [14].
Figure 1: Pre iPDT Contrast-Enhanced (CE) and post iPDT Non-Contrast-En- hanced (NCE) T1-weighted MRI for two examples having largest (#9 OV=0.99) and smallest overlap (#10 OV=0.02); MRI-based segmentation of the tumor (red) and the intrinsic T1-hyperintensity (blue) in the NCE-T1 post iPDT MRI; 3D superimposition of volumes with visualization of the diffuser parts of the cylindrical-diffuser-fibers (black); adapted from [5].
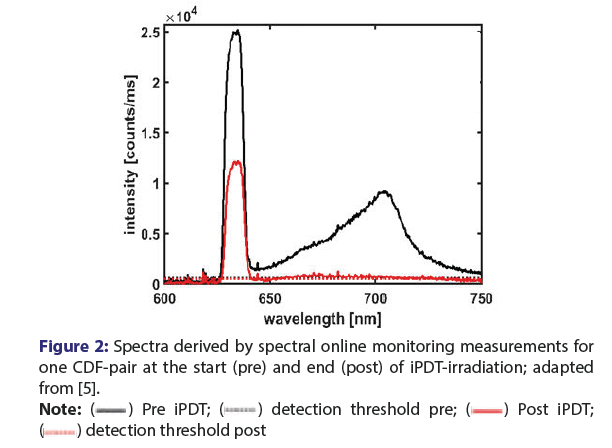
Figure 2: Spectra derived by spectral online monitoring measurements for
one CDF-pair at the start (pre) and end (post) of iPDT-irradiation; adapted
from [5]. 

The volumetric analysis showed that an intrinsic T1-hyperintensity volume (median volume 0.79 cm3, range: (0.05 cm3-3.93 cm3)) occurred after each iPDT-irradiation. The measured volumes are comparable in size and showed similarities to asymptomatic “silent” haemorrhages [15]. These haemorrhages can arise after biopsy or needle insertion into the brain by direct injury to capillaries vessels or shearing forces in the tissue [16-19].
The 3D overlap showed that the T1-hyperintensity volume intersects with the initial tumor volume (median overlap 0.61, range (0.02-0.99)). All these T1-hyperintensities can be interpreted as iPDT-related as they are localized either within or immediately surrounding of the tumor volume. The surrounding area is known as the diffuse infiltration zone of the malignant glioma having no or minor contrast uptake [20].
Spectral Online Monitoring (SOM)
The spectrally resolved data are acquired via laser irradiation through one CDF and detection of the transmitted treatment light and the PpIX fluorescence signal by means of a spectrometer connected to another CDF [21,22]. Employing this technique, spectral measurements can be performed at different time points (Figure 2) [5,7,21,22]. Measurement before iPDT-illumination gives information about the presence of PpIX fluorescence (local maximum at 700 nm, black) and thus confirms that the photosensitizer is available within the target volume. In addition, laser light transmission (local maximum at 635 nm) between two CDFs can be assessed.
Furthermore, illumination light transmission and PpIX fluorescence can be assessed also during and at the end of iPDT-illumination [22]. Due to photo bleaching, after iPDT-illumination, there should be a complete loss of the PpIX-fluorescence signal. Case wise, a change in the transmission can also be observed (see Figure 2 red line) [5,7]. The first analysis of SOM data showed that changes in the treatment light transmission signals might be observed during iPDT in addition to the expected bleaching of the fluorescence. These observations included significant decreases or total losses of the treatment light transmission signal [22].
A deeper analysis of SOM data was performed to extract the optical absorption coefficient µa at the treatment wavelength of 635 nm. therefore, an equation adopted from the diffusion approximation was utilized [23,24] to calculate theoretical signal intensities between two CDFs in warped position to each other for a given set of µa-values, assuming a pre-set reduced scattering coeficient of µs’=2 mm-1 and homogeneous tissue [5]. Out of the comparison of theoretical with measured signals, the local µa-value can be derived. This µa-value only gives an average measure of the optical absorption in the tissue surrounding the CDF-pair, as the assumption of homogeneous tissue does not represent the proper tissue morphology and includes the effects of in homogeneities in the tissue on the signals. In the end, the determined average µa-values (115 analysable CDF-pairs; median μa = 0.068 mm-1, IQR: (0.045 0.093)) are well consistent with the range of absorption values found in the literature ({0.02-0.08} mm-1) [25,26]. In combination with calculated µa-values at the end of the iPDTirradiation, it allows the determination of the approximate change in absorption in the area surrounding the CDF-pair. This change showed an increase in absorption in 85% of the analysable CDF-pairs. Larger increases in absorption can theoretically not be only caused by oxygenated haemoglobin [5]. As deoxygenated haemoglobin and methaemoglobin have much higher absorption than oxygenated haemoglobin, 8.5 times and 33 times higher, respectively, a generation of these haemoglobin species must be considered for clinical iPDT [12,27,28].
Correlation and context between SOM and T1- hyperintensity
Both laboratory experiments and the known progress of blood degradation in a hemorrhage in MRI indicate that the mentioned observations in the clinical data of SOM (decrease of the transmission signal, respectively, increase in tissue absorption) and in the MRI (occurrence of a T1-hyperintensity) cannot be associated only with the presence of blood. An iPDT-irradiation-induced change in the haemoglobin species has to be considered by deoxygenation and even possible oxidation of haemoglobin [12]. For this reason, an exploratory analysis was conducted in which a correlation between the transmission/ absorption change and the localization of T1-hyperintensity was performed [5]
Herein a 3-dimensional light zone was defined around each CDFpair to link the SOM data and the MRI. This light zone represents the volume in which at least 67% of the light paths of the detected photons are localized based on light distribution calculations. For comparison, the light zone was individually created for each diffuser pair using CAD and then superimposed with the intrinsic T1-hyperintensity. A CDFpair was considered influenced by T1-hyperintensity if an intersection was present so that a distinction could be made between CDF-pairs with T1-hyperintensity localization and without. The comparison of µa change with and without T1-hyperintensity influence showed a significant correlation (p=0.003) [5]. there are also indications of a tendency towards a higher µa increase when the intersection volume between the light zone and T1-hyperintensity is larger.
Discussion
The described correlation between the absorption increase, detectable by treatment light transmission signal decrease of SOM, and the observation of the intrinsic T1-hyperintensity in the MRI is based on the post-iPDT one day after therapy which is usually not expected. Typically, the occurrence of T1-hyperintensity is observed only occasionally for haemorrhages until 48 h after the bleeding [14,29-31]. After iPDT, however, this observation is made for all cases within one day so far [13]. this suggests that the iPDT-related oxygen consumption and the development of ROS may accelerate the transformation of haemoglobin from the oxygenated to the deoxygenated state and further to the oxidation to methaemoglobin in a haemorrhage.
Unfortunately, the MRI is acquired the next day. therefore, an earlier determination of a haemorrhage volume after an iPDT cannot be performed. In the early post-therapeutic MRI, only the haemoglobin volume so far oxidized to methaemoglobin is visible. It is unknown which portion was (faster) converted following iPDT-effects (deoxygenation by ROS production and so faster conversion to methaemoglobin or direct oxygenation by ROS) [12] or which portion was potentially converted due to upcoming inflammatory reactions in the treated volume [29]. Only CT/MRI imaging immediately after iPDT-irradiation can provide insight, but such imaging is not performed without clinical necessity.
It must be mentioned that a haemorrhage’s occurrence may not directly lead to a change in light dosimetry. Laboratory experiments have shown that significant changes in light penetration by the genesis of methaemoglobin may occur towards the iPDT irradiation end at about 40 min after irradiation start [12]. Online monitoring measurements showed that after this time, most of the PpIX may already be photo bleached, visible by the decrease in PpIX fluorescence [22]. So the main phototoxic effect may have already taken place.
Up to now, it remains unknown if and how the occurrence of a silent haemorrhage affects the iPDT outcome. Interestingly, analysis of SOM-data acquired during iPDT interventions on malignant glioma recurrences showed that more stable optical conditions, meaning less change or reduction in the measured treatment light transmission signal, can be associated with prolonged patient survival [7]. This indicates that the use of SOM and its evaluation may potentially be used as a prognostic indicator in addition to direct monitoring of fluorescence and optical stability. Further in-depth investigations and research should be performed to support and manifest such speculation.
Conclusion
Investigations on SOM and its relation to MRI result in more insight into observations made regarding clinical iPDT. The observed relations between the two phenomena, reduction in treatment light transmission during iPDT and post-therapeutic observation of T1-hyperintensity, might essentially be explained by a relatively rapid iPDT-induced deoxygenation of haemoglobin and genesis of methaemoglobin. The knowledge gained and the methods developed can serve as a basis for further research to improve iPDT irradiation concepts and individualize iPDT based on the optical tissue properties obtained.
Acknowledgments
Funding
This research was supported by the German Research Foundation (DFG) in terms of the Research Training Group (RTG) GRK2274.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Ludwig- Maximilians-University, Munich, Germany (reference number 335-16).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Disclosure
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rasmussen BK, Hansen S, Laursen RJ, et al. Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I-IV in the the Danish Neuro-Oncology Registry. J Neurooncol. 2017;135:571-579.
[Crossref] [Google Scholar] [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466.
[Crossref] [Google Scholar] [PubMed].
- Muller DMJ, Robe PA, Ardon H, et al. Quantifying eloquent locations for glioblastoma surgery using resection probability maps. J Neurosurg. 2020;134:1091-1101.
[Crossref] [Google Scholar] [PubMed].
- Stepp H, Stummer W. 5-ALA in the management of malignant glioma. Lasers Surg Med. 2018;50(5):399-419.
[Cross Ref] [Google Scholar] [PubMed].
- Aumiller M, Heckl C, Quach S, et al. Interrelation between Spectral Online Monitoring and Postoperative T1-Weighted MRI in Interstitial Photodynamic Therapy of Malignant Gliomas. Cancers. 2021;14(1):120.
[Crossref] [Google Scholar] [PubMed].
- Beck TJ, Kreth FW, Beyer W, et al. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med. 2007;39(5):386-393.
[Crossref] [Google Scholar] [PubMed].
- Lietke S, Schmutzer M, Schwartz C, et al. Interstitial photodynamic therapy using 5-ala for malignant glioma recurrences. Cancers. 2021;13(8):1767.
[Crossref] [Google Scholar] [PubMed].
- Jacques SL. How tissue optics affect dosimetry of photodynamic therapy. J Biomed Opt. 2010;15(5):051608.
[Crossref] [Google Scholar] [PubMed].
- Jacques SL. Optical properties of biological tissues: A review. Phys Med Biol. 2013;58(11):37-61.
[Crossref] [Google Scholar] [PubMed].
- Gonçalves TM, Martins IS, Silva HF. Spectral optical properties of rabbit brain cortex between 200 and 1000 nm. Photochem. 2021;1(2):190-208.
- Prahl S. Optical absorption of hemoglobin. 1999.
- Heckl C, Aumiller M, Rühm A, et al. Fluorescence and treatment light monitoring for interstitial photodynamic therapy. Photochem Photobiol. 2020;96(2):388-96.
[Crossref] [Google Scholar] [PubMed]
- Aumiller M, Ertl-Wagner B, Heckl C, et al. Investigation on treatment light transmission changes during IPDT in comparison to T1 hyperintensity in early post-therapeutic T1-weighted MRI. J Opt Soc Am. 2021.
- Bradley Jr WG. MR appearance of hemorrhage in the brain. Radiology. 1993;189(1):15-26.
[Crossref] [Google Scholar] [PubMed]
- Kulkarni AV, Guha A, Lozano A et al. Incidence of silent hemorrhage and delayed deterioration after stereotactic brain biopsy. J Neurosurg. 1998;89(1):31-5.
[Crossref] [Google Scholar] [PubMed]
- Casanova F, Carney PR, Sarntinoranont M. Effect of needle insertion speed on tissue injury, stress, and backflow distribution for convection-enhanced delivery in the rat brain. PLoS One. 2014;9(4):e94919.
[Crossref] [Google Scholar] [PubMed]
- Casanova F, Carney PR, Sarntinoranont M. In vivo evaluation of needle force and friction stress during insertion at varying insertion speed into the brain. J Neurosci Methodss. 2014;237:79-89.
[Cross Ref] [Google Scholar] [PubMed]
- Kreth FW, Muacevic A. Stereotactic biopsy and hemorrhage. J Neurosurg 1999; 181-182.
[PubMed]
- Kreth FW, Muacevic A, Medele R, et al. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours–a prospective study. Acta Neurochir. 2001;143(6):539-46.
[Cross Ref] [Google Scholar] [PubMed]
- Schucht P, Knittel S, Slotboom J, et al. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2014;156(2):305-12.
[Crossref] [Google Scholar] [PubMed]
- Johansson A, Faber F, Kniebühler G, et al. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg Med. 2013;45(4):225-34.
[Crossref] [Google Scholar] [PubMed]
- Rühm A, Stepp H, Beyer W, et al. 5-ALA based photodynamic management of glioblastoma. Neurosurg, Neurophotoni, and Optogen 2014.
- Martelli F. Light propagation through biological tissue and other diffusive media: Theory, solutions, and software. SPIE press; 2009.
- Wang LV, Wu HI. Biomedical optics: Principles and imaging. JWS. 2012.
- Gebhart SC, Lin WC, Mahadevan-Jansen A. In vitro determination of normal and neoplastic human brain tissue optical properties using inverse adding-doubling. Phys Med Biol. 2006;51(8):2011.
[Crossref] [Google Scholar] [PubMed]
- Yaroslavsky AN, Schulze PC, Yaroslavsky IV, et al. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys Med Biol. 2002;47(12):2059.
[Crossref] [Google Scholar] [PubMed]
- Larsen EL, Randeberg LL, Gederaas OA, et al. In vitro study on methemoglobin formation in erythrocytes following hexyl-aminolevulinate induced photodynamic therapy. SPIE. 2007.
- Larsen EL, Randeberg LL, Gederaas OA, et al. Monitoring of hexyl 5-aminolevulinate-induced photodynamic therapy in rat bladder cancer by optical spectroscopy. J Biomed Opt.. 2008;13(4):044031.
[Crossref] [Google Scholar] [PubMed]
- Hasan D, Nikoubashman O, Pjontek R, et al. MRI appearance of chronic subdural hematoma. Front Neurol. 2022.
[Google Scholar] [PubMed]
- Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. Lancet Neurol. 2008;7(3):256-67.
[Crossref] [Google Scholar] [PubMed]
- Anzalone N, Scotti R, Riva R. Neuroradiologic differential diagnosis of cerebral intraparenchymal hemorrhage. Neurol Sci. 2004;25(1):3-5.
[Crossref] [Google Scholar] [PubMed]