In vitro availability of chloramphenicol in the presence of cadmium and lead salts
- *Corresponding Author:
Date of Received: 25-02-2010
Date of Modified: 22-03-2010
Date of Accepted: 15-04-2010
Available Online: 15-05-2010
Abstract
Metal ions have been reported to form chelate complexes with certain drug molecules especially those that contain ligand donor atoms. In this study, the in vitro availability of chloramphenicol in the presence of Pb and Cd was evaluated. These studies were carried out in simulated gastric juice (0.1M HCl) and intestinal pH (pH 9) at 37°C over period of 180 minutes. A double scanning UV/VIS spectrophotometer (Helios Zeta, Model 164617) was used to analyze drug content by measuring absorbance at 278 nm. An overall chloramphenicol availability of 89, 88.6 and 86% was achieved in simulated gastric juice for chloramphenicol alone, in the presence of Pb, and of Cd respectively. The corresponding availabilities observed in simulated intestinal pH were 82.5, 63.2 and 63.2%. The presence of Pb and Cd did not significantly affect the overall availability of chloramphenicol in simulated gastric juice. However, the availability of the drug was significantly decreased by Pb and Cd in simulated intestinal pH. The results of this study indicate that the availability of chloramphenicol may be reduced in the presence of Pb and Cd especially from the intestine, which may result in therapeutic failure.
Keywords
Chloramphenicol, in vitro availability, Lead, Cadmium.
Introduction
Chloramphenicol is 2,2-dichloro-N-[(1R, 2R)-2-hydroxy-1-(hydroxymethyl)-2-(4- nitrophenyl)ethyl]-acetamide (Figure 1), it was originally isolated as a substance produced by certain strains of the soil organism Streptomyces venezuelae, but is now produced synthetically. [1] Chloramphenicol usually acts as a bacteriostatic antibiotic with a broad spectrum of action against both Gram-positive and Gram-negative bacteria, as well as some other organisms, but at higher concentrations or against some very susceptible organisms it can be bactericidal. [1] It is used in the treatment of human infection with Salmonella typhi (typhoid) and other forms of salmonellosis, and other lifethreatening infections of the central nervous system and respiratory tract. [2] In veterinary medicine, chloramphenicol is used for the treatment of a variety of infections in animals, particularly those caused by anaerobic bacteria or those that are resistant to other antimicrobial agents. Chloramphenicol in animals is well absorbed by both oral and parenteral routes. [3] The risk of life threatening adverse effects (particularly bone marrow aplasia) and resistance, has severely limited the clinical usefulness of chloramphenicol, although it is still widely used in some countries (including Nigeria). [1]
Metal complexes with active pharmaceuticals in which the drug molecules play the role of a ligand have been reported. [4-7] Chloramphenicol – metal complexes with Zn, Cd, Sn and Pb have been synthesized, with the spectral data of the complexes indicating deprotonation and coordination of the secondary alcoholic OH group with metal ions, but the amide group does not participate in coordination. [8] The nitro group has also been demonstrated to be involved in coordination in a chloramphenicol – Ni. [9] As a result of such interactions, metal ions have been reported to significantly alter the absorption and consequently the bioavailability of different drugs especially antibiotics. [10-12] This study was therefore aimed at determining the in vitro availability of chloramphenicol in simulated gastric and intestinal media in the presence of Cd and Pb, two metals that are commonly found as contaminants in drinking water, food, drugs and environmental samples.
Material and Methods
Equipment and reagents
Chloramphenicol reference standard was obtained as a gift from Doyin Pharmaceuticals Nigeria Ltd. Chloramphenicol capsules were products of Green Field Pharmaceutical (JIANGSU) Co. Ltd., China (Batch-04-5485). The metal nitrates and other chemical reagents used were of analytical grade. Dissolution studies were performed using a ERWEKA (GMBH) dissolution apparatus. A double scanning UV/VIS spectrophotometer (Helios Zeta, Model 164617) was used to monitor the drug content.
Identification and assay of chloramphenicol
Both the chloramphenicol reference standard and chloramphenicol capsules were identified and assayed using official methods. [13]
Preparation of dissolution media
Dissolution was carried out in simulated gastric juice (containing 0.1M hydrochloric acid) and intestinal pH (phosphate buffer pH 9). Both media were prepared using official methods. [13]
Preparation of calibration curve
Stock solutions (1 mg/ml) of chloramphenicol reference standard were prepared in 0.1M hydrochloric acid and phosphate buffer pH 9. The absorbance of a 50 μg/ml solution was measured in the range of 200 – 350 nm, which showed maxima at 278 nm. Successive serial dilutions of the stock were done to obtain standard solutions of concentration 10, 20, 30, 40 and 50 μg/ml. The absorbance of each solution was measured at the maxima (278 nm). [13] A plot of concentration against the corresponding absorbance was done in the case of each media. A straight line was obtained which obeyed the Beer-Lambert’s law in each case. The chloramphenicol content in the two media during the in vitro availability studies were extrapolated from the calibration curves.
Availability of chloramphenicol
The in vitro availability of chloramphenicol in simulated gastric juice and intestinal pH at 37°C was determined using dissolution apparatus as outlined in BP (2002) [13] with slight modification to the top of the basket in order to prevent air entrapment during dissolution. [14] A capsule of chloramphenicol (250 mg) was placed in the dissolution basket and allowed into the dissolution medium. The dissolution process was monitored over a period of 180 minutes, with aliquots (10 ml) being withdrawn at 15 minutes intervals and assayed for drug content available. The volume of the dissolution medium was maintained after each withdrawal by an immediate replacement with 10 ml of the dissolution medium maintained at the same temperature in the same bath.
For the in vitro availability of chloramphenicol in the presence of Cd and Pb, the drug and the nitrate salts of each of the metals were reacted in a ratio of 2:1 (i.e 250 mg chloramphenicol:125 mg of metal) respectively in each of the dissolution media (1L). The process described above was then repeated to determine the drug content after each sampling.
Statistical Analysis
The results obtained were statistically analyzed using student t-test with GraphPad Prism 4 software. P values less than 0.05 were considered to be statistically significant.
Results and Discussion
The results of the identification test for both chloramphenicol reference standard and capsules revealed the presence of the active ingredient, and the content assayed was within the official limits (98 – 105%). [13] For the quantitative determination of drug content, it was observed that the calibration curves of chloramphenicol in both simulated gastric juice (0.1M hydrochloric acid) and intestinal pH (phosphate buffer pH 9) at 278 nm obeyed the Beer–Lambert’s law. The linear relationship between absorbance (A) and concentration (C in % w/v) is given by the regression equations A = 0.030C + 0.003 and A = 0.033C + 0.002 for chloramphenicol in 0.1M hydrochloric acid and phosphate buffer pH 9 respectively. The coefficient of correlation (r) in each case is 0.999. The calculated molar absorptivities were 1,061 ± 0.0004 M-1 cm-1 and 1,125 ± 0.0003 M-1 cm-1 for 0.1M hydrochloric acid and phosphate buffer pH 9 respectively.
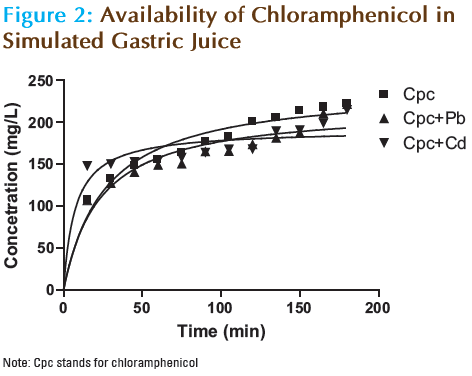
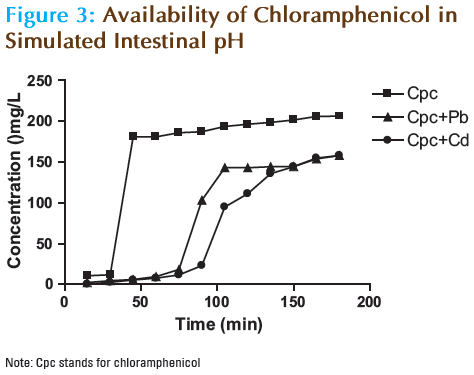
The results of the in vitro availability of chloramphenicol at different time intervals in simulated gastric juice and intestinal pH at 37°C are shown in Tables 1 and 2 as well as in Figures 2 and 3. The intrinsic first order dissolution rate constants and dissolution times T50% and T90% of chloramphenicol alone and in the presence of Pb and Cd are shown in Table 3.
| Time (min) | Chloramphenicol | Chloramphenicol + Pb | Chloramphenicol + Cd | ||||
|---|---|---|---|---|---|---|---|
| Conc. (mg/l) | % Conc. | Conc. (mg/l) | % Conc. | Conc. (mg/l) | % Conc. | ||
| 15 | 108.3 | 43.3 | 107.0 | 42.8 | 148.3 | 59.3 | |
| 30 | 133.0 | 52.2 | 127.3 | 50.9 | 150.6 | 60.2 | |
| 45 | 148.6 | 59.4 | 140.6 | 56.4 | 153.0 | 61.2 | |
| 60 | 156.0 | 62.4 | 149.0 | 56.6 | 154.6 | 61.8 | |
| 75 | 164.0 | 65.6 | 150.6 | 60.2 | 158.3 | 63.3 | |
| 90 | 177.3 | 70.9 | 165.0 | 66.0 | 164.3 | 65.7 | |
| 105 | 182.6 | 73.0 | 165.6 | 66.2 | 168.0 | 67.2 | |
| 120 | 200.3 | 80.1 | 173.6 | 69.4 | 168.3 | 67.3 | |
| 135 | 205.3 | 82.1 | 181.3 | 72.5 | 189.6 | 73.8 | |
| 150 | 214.3 | 85.7 | 187.0 | 74.8 | 191.0 | 76.4 | |
| 165 | 218.3 | 87.3 | 210.3 | 84.1 | 199.3 | 79.7 | |
| 180 | 222.6 | 89.0 | 221.6 | 88.6 | 215.0 | 86.0 | |
Table 1: Availability of chloramphenicol at different time intervals in simulated gastric juice at 37°C
| Time (min) | Chloramphenicol | Chloramphenicol + Pb | Chloramphenicol + Cd | |||
|---|---|---|---|---|---|---|
| Conc. (mg/l) | % Conc. | Conc. (mg/l) | % Conc. | Conc. (mg/l) | % Conc. | |
| 15 | 10.5 | 4.2 | 2.2 | 0.9 | 0.4 | 0.2 |
| 30 | 11.8 | 4.7 | 4.5 | 1.9 | 2.7 | 1.1 |
| 45 | 181.0 | 72.6 | 5.9 | 2.4 | 5.3 | 2.1 |
| 60 | 181.0 | 72.6 | 9.5 | 3.8 | 7.6 | 3.0 |
| 75 | 186.0 | 74.4 | 18.2 | 7.3 | 11.6 | 4.6 |
| 90 | 187.2 | 74.9 | 103.3 | 41.3 | 23.1 | 9.2 |
| 105 | 193.6 | 77.4 | 143.0 | 57.2 | 94.8 | 37.9 |
| 120 | 196.3 | 78.5 | 143.0 | 57.2 | 111.2 | 44.5 |
| 135 | 198.7 | 79.5 | 144.2 | 57.7 | 135.7 | 54.3 |
| 150 | 201.5 | 80.6 | 144.5 | 57.8 | 144.2 | 57.7 |
| 165 | 206.0 | 82.4 | 153.9 | 61.6 | 154.8 | 61.9 |
| 180 | 206.3 | 82.5 | 158.0 | 63.2 | 158.0 | 63.2 |
Table 2: Availability of chloramphenicol at different time intervals in simulated intestinal pH at 37°C
| Simulated Gastric Juice | Simulated Intestinal pH | |||||
|---|---|---|---|---|---|---|
| T50% | T90% | K | T50% | T90% | K | |
| Chloramphenicol | 28.7 | 182.0 | 0.06 | 31.0 | 196.4 | 0.09 |
| Chloramphenicol+Pb | 29.5 | 182.8 | 0.06 | 91.8 | 256.3 | 0.20 |
| Chloramphenicol+Cd | 12.6 | 188.4 | 0.08 | 124.3 | 256.3 | 0.20 |
T50% and T90%: time (minutes) for availability 50 and 90% of the drug respectively
K: first order dissolution rate constant (min-1) of the drug
Table 3: First Order Dissolution Rates of Chloramphenicol at 37°C
In simulated gastric juice at 37°C, chloramphenicol achieved an availability of 89% after 180 minutes, with T50% and T90% values of 28.7 and 182 minutes respectively. In the presence of Pb and Cd, c hloramphenicol achieved an availability of 88.6 and 86% after 180 minutes. The overall availability of chloramphenicol in simulated gastric juice at 377°C was not significantly affected (P<0.05) by the presence of the metals, although the drug content available after each sampling was affected. The consistent decline in the content of chloramphenicol available in the presence of Pb throughout the study did not however significantly change (P<0.05) the T50% (29.5 minutes) and T90% (182.8 minutes) values. Cd appears to increase the availability of chloramphenicol for the first 45 minutes resulting in significant reduction (P<0.05) of the T50% (12.6 minutes). Thereafter a decline in drug content was observed to the end of the study resulting in an increased T90% (188.4 minutes) value. A similar trend was observed in in vitro availability and interaction studies of ciprofloxacin and ofloxacin in the presence of some metals. [11,15] The insignificant reduction in drug content observed in the presence of Pb and Cd in the simulated gastric juice may be because the complexation of certain drugs by polyvalent cations is not favored in acid medium. [15]
In the simulated intestinal pH, chloramphenicol achieved an availability of 82.5% after 180 minutes, with T50% and T90% values of 31.0 and 196.4 minutes respectively. In presence of Pb and Cd, the content of chloramphenicol available was drastically reduced to 63.2% in both cases. The overall availability of chloramphenicol in simulated intestinal pH at 37°C was significantly reduced in the presence of Pb (P<0.01), as well as in the presence of Cd (P<0.001). The consistency and degree of decline in the content of chloramphenicol available with time in the presence of both Pb and Cd was underscored by the significant change (P<0.01) in both the T50% and T90% values. The T50% values where increased from 31.0 minutes to 91.8 and 124.3 minutes for chloramphenicol in the presence of Pb and Cd respectively. There was also a significant increase (P<0.01) in the T90% values from 196.4 minutes to 256.3 minutes in both cases.
It is evident from the results obtained that the effects of Pb and Cd on the in vitro availability of chloramphenicol are more pronounced in simulated intestinal pH. This may be attributed to reports suggesting that the dissolution rate of certain antibiotics is markedly reduced at high pH values. [16] Another reason could be the complexation of the drug by the metal resulting in reduced availability due to an increase in T50% and T90% values. A similar trend was also reported where the availability of ofloxacin was markedly reduced in the presence of different metals (including Cd) in a medium of pH 9 at 37°C. [11]
Conclusion
In this study, the in vitro availability of chloramphenicol was reduced in the presence of Pb and Cd. This effect was more significant in simulated intestinal pH than in simulated gastric juice in terms of magnitude and linearity. Furthermore, the pH of the medium is an important factor in the dissolution and consequent availability of the drug. Thus, the concurrent use of chloramphenicol with water, food and drugs containing Pb and Cd metal ions may result in decreased absorption, decreased serum concentration and decreased bioavailability of chloramphenicol, leading to therapeutic failure. Further studies on the effect of actual concentrations of these metals found as contaminants in water, food and drugs on the bioavailability of chloramphenicol should be evaluated.
References
- Sweetman, S.C. (Ed), Martindale: The Complete Drug Reference, 34th Ed., London: Pharmaceutical Press. Electronic version, 2005.
- Parfitt, K. (Ed), Martindale: The Complete Drug Reference, 32nd Ed., London: Pharmaceutical Press, 1999, pp. 182 – 184.
- Plumb, D.C. Veterinary Drug Handbook, 4th Ed., Ames: Iowa State Press, 2002, pp. 166 – 169.
- Eboka, C.J and Okeri, H.A. Aqueous Solubility of Ciprofloxacin in the Presence of Metal Cations. Trop Jour Pharm Res 2005; 4 (1): 349 – 354.
- Edrissi, M., Razzaghi Asl, N and Madjidi, . Interaction of Mefenamic Acid with Cobalt (II) Ions in Aqueous Media: Evaluation via Classic and Response Surface Methods. Turk Jour Chem 2008; 32 : 505 – 519.
- Zaman, M.K, Arayne, M.S, Sultana, N And Farooq, A. Synthesis And Characterization of Glibenclamide Complexes of Magnesium, Chromium, Cobalt, Nickel, Zinc and Cadmium Salts. Pak. J. Pharm. Sci., 2006; 19 (2): 114 – 118.
- Wisniewski, M.Z, Glowiak, T, Opolski, A and Wietrzyk, J. Synthesis, Characterization And Antiproliferative Activity of the Co(II), Ni(II), Cu(II), Pd(II) and Pt(II) Complexes of 2-(4-Thiazolyl) Benzimidazole (Thiabendazole). Metal Based Drugs, 2001; 8 (4): 189 – 194.
- Mishra, P, Singh, B and Sharma, R. Synthesis, Spectroscopy, Characterization and Molecular Modeling of Biologically Active Chloramphenicol with Main Group Ions. Main Group Chem, 2007; 6 (1): 31 – 41.
- Guru, P. Studies of Nickel Compound with Chloramphenicol. Int Jour ChemTech Res, 2009; 1 (3): 552 – 554.
- Arayne MS, Sultana N and Hussain F. Interaction between Ciprofloxacin and Antacids. Adsorption and Dissolution Studies. Drug Met.& Drug Inter, 2005; 21(2): 117 – 129.
- Sultana, N, Arayne, M.S and Yasmeen, N. In Vitro Availability of Ofloxacin in Presence of Metals Essential to Human Body. Pak. J. Pharm. Sci., 2007; 20 (1): 36 – 42.
- Flor, S., Guay, D.R.P and Opsahl, J.A. Effect of magnesium- aluminum hydroxide and calcium carbonate antacids on the bioavailability of ofloxacin. Antimicrob. Agents Chemother., 1991; 34: 2436 – 2438.
- British Pharmacopoeia (2002) Vol. II. Printed for Her Majesty’s Stationery Office at University Press, Cambridge, Appendix VII.
- Arayne, M.S, Sultana, N and Kamran Zaman, R.M. In Vitro Availability of Glibenclamide in Presence of Antacids. Pak. J. Pharm. Sci., 2004; 17 (2): 41 – 56.
- Sanchez, B.M, Cabarga, A.S, Navario, A.S and Hurle, A.D. A Physicochemical Study of the Interaction of Ciprofloxacin and Ofloxacin with
- Polyvalent Cations. Int.J. Pharmaceutics, 1994; 106: 229 – 235. Sultana, N., Arayne, M.S and Ghazali, F.A. Effects of Antacids on the Dissolution Behavior of Methacycline and Doxycycline. J.P.M.A., 1984; 34 (3): 59 – 63.