In vitro antioxidant activity of Holarrhena antidysenterica Wall. methanolic leaf extract
- *Corresponding Author:
- P. S.Sujan Ganapathy
Centre for Vrikshayurveda, A Division of Centre for Advanced Studies in Biosciences, Jain University, Chamrajpet- 560019, Karnataka, India.
E-mail: sujan.ganapathy@gmail.com
Date of Received : 14-04-2011
Date of Accepted : 18-05-2011
Available Online : 15-11-2011
Abstract
Antioxidative potential of methanolic leaf extract of Holarrhena antidysenterica was evaluated using hydroxyl radical, superoxide anion scavenging and reducing power assays. The antioxidant activity of the methanol extract increased in a concentration-dependent manner. The extract showed significant reactive oxygen species (ROS) scavenging activity in all in vitro antioxidant assays and contained high level of total phenolic content.
Keywords
Medicinal plant, antioxidant, reactive oxygen species, nutraceutical
Introduction
Reactive oxygen species (ROS), which consist of free radicals such as superoxide anion (O2·_) and hydroxyl (HO·) radicals and non-free radical species such as H2O2 and singled oxygen (O2), are different forms of activated oxygen [1,2,3]. ROS are produced by all aerobic organisms and can easily react with most biological molecules including proteins, lipids, lipoproteins and DNA. Thus, ample generation of ROS proceed to a variety of pathophysiological disorders such as arthritis, diabetes, inflammation, cancer and genotoxicity [4,5]. Therefore, living organisms possess a number of protective mechanisms against the oxidative stress and toxic effects of ROS.
Antioxidants regulate various oxidative reactions naturally occurring in tissues and are evaluated as a potential anti-aging agent. Hence, antioxidants can terminate or retard the oxidation process by scavenging free radicals, chelating free catalytic metals and also by acting as electron donors. Antioxidants have been widely used as food additives to provide protection from oxidative degradation of foods and oils. Hence, antioxidants are used to protect food quality mainly by the prevention of oxidative deterioration of constituents of lipids. The most extensive- ly used synthetic antioxidants are propylgallate (PG), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tert-butylhydroquinone (TBHQ) [6]. However BHT and BHA have been suspected of being responsible for liver damage and carcinogenesis [7-9]. Natural antioxidants are able to protect from ROS as well as other free radicals and retard the progress of many chronic diseases and lipid oxidative rancidity in foods [10-13].
Holarrhena antidysenterica Wall. (Apocynaceae), commonly known as “Kutaja”, is an important plant used in indigenous systems of medicine as remedy for bronchitis, hematuria, spermatorrhoea, epilepsy, asthma, piles, leprosy, eczema, diarrhea, fevers and jaundice [14,15]. The bark has been reported to possess astringent and antidiarrheal properties [16]. Leaves of the plant are used to cure scabies [17]. In addition, the leaves of H. antidysenterica have been reported to have antibacterial, anti-inflammatory and analgesic activity [18,19].
Nevertheless, there is as yet no report concerning the antioxidant effect of this plant. Our main objective is to determine the potential natural antioxidant source. However, the purpose of this particular study was to evaluate the in vitro antioxidant activities of methanol extract of H. antidysenterica.
Materials and Methods
Plant materials
Fresh material of plant in the flowering stage above 20 cm diameter at breast height were collected locally from Bhadra Wild Life Sanctuary, Karnataka (Southern India) in May 2006. The taxonomic identification of the plant was confirmed by Dr. Y. L. Ramachandra, Department of Biotechnology, Kuvempu University, Shankaraghatta (Voucher specimen number YLR204).
Extraction
Freshly collected leaves of H. antidysenterica were shade-dried and then powdered using a mechanical grinder. 250 grams of pulverized leaf material were soaked in 750 ml of methanol (LR grade, Merck, India) and kept on a rotary shaker for 24 h. Each extract was filtered under vacuum through a Whatman No.1 filter paper and the process repeated until all soluble compounds had been extracted. Extraction was considered to be complete when the filtrate had a faint colour. The extracts were evaporated to dryness under reduced pressure using a Rotavapor (Buchi Flawil, Switzerland). A portion of the residue was subjected to screening for antioxidant activity.
Antioxidant Activity
Superoxide anion scavenging activity
Superoxide anion scavenging capacity of H. antidysenterica was assessed by the method of Nishikimi [20]. About 1 mL of reaction mixture contained 156 μM nitoblue tetrazolium in 100 mM phosphate buffer (pH 7.4), 468μM NADH in 100 mM phosphate buffer (pH 7.4) with 0.1 mL of extract (200μg to 1000μg as equivalents to tannic acid) in methanol were mixed. The reaction started by add- ing 100 μL of 60 μM phenazine methosulphate solution in 100mM phosphate buffer (pH 7.4) to the mixture. The reaction mixture was incubated at 25°C for 5 min, correspondingly blank contained all the reagents except extract of H.antidysenterica and the absorbance of oxidized product formazan was recorded at 560 nm. Decreased absorbance of the reaction mixture indicated increased superoxide anion scavenging activity. The percentage of super oxide anion scavenging was cal- culated as:
% scavenged of superoxide anion = [(Ablank−ASample) /Ablank] × 100
where Ablank is the absorbance of the blank in absence of sample, and ASample is the absorbance in the presence of the sample.
Hydroxyl radical scavenging assay
The ability of the extract of H.antidysenterica to scavenge the hydroxyl radical being generated by Fenton reaction Fe3+–ascorbate–EDTA–H2O2 system was performed by the method of Halliwell et al. [21] with minor changes. Freshly prepared 1.0ml of final reaction mixture containing 100μl of 28mM 2-deoxyribose in phosphate buffer (pH 7.4), 500μl of various concentrations of (100–500μg/ml) H.antidysenterica extract, 200μl of 200μM FeCl3 in 1.04mM EDTA (1:1, v/v), 100μl each of 1.0mM H2O2 and ascorbic acid, respectively. After 1hr of incubation at 37°C the free radical damage imposed on deoxyribose was measured as TBARS by the method of Ohkawa et al. [22] 1.0 ml thiobarbituric acid (1%) and 1.0ml of trichloro acetic acid (2.8%) were added to the test tubes and were incubated in boiling water bath for 20min. After cooling, absorbance was measured at 532nm against the blank containing deoxyribose and buffer. The percentage of hydroxyl radical scavenging was calculated as:
% Scavenged [H2O2 ] = [(Ablank−ASample) /Ablank] × 100
where, Ablank is the absorbance of the blank in absence of sample, and ASample is the absorbance in the presence of sample.
Fe3+- Reducing power assay
The Fe3+-reducing power was determined using the method described by Oyaizu [23]. Different concentrations (50–250μg as equivalents to tannic acid) of H.antidysenterica extract in 1ml of methanol were mixed with 2.5 ml of phosphate buffer (200mM, pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was placed in a water bath for 20 min at 50°C, then cooled rapidly and mixed with 2.5 ml of 10% trichloroacetic acid. The resulting solution was centrifuged at 3000 rpm for 10 min. From the supernatant 2.5ml of fraction was mixed with 2.5ml of distilled water and 0.5 ml of 0.1% ferric chloride and the amount of iron(II)-ferricyanide complex could be determined by measuring the formation of Perl’s Prussian blue at 700 nm after 10 min. The higher absorbance of the reaction mixture indicates increased reducing power.
Total phenolic assay
Total phenolic in the methanolic leaf extract of H.antidysenterica was determined according to the method of Shahidi and Naczk [24]. An amount of 0.25 ml aliquot of the prepared samples were mixed with 0.25 ml Folin-Ciocalteu reagent (previously diluted with water 1:1 (v/v)) and 0.5ml of saturated sodium carbon- ate (Na2CO3) solution and 4ml of deionized water. The mixtures were intensively shaken, left at room temperature for 25 min and centrifuged at 5000 rpm for 10 min. the supernatant absorbance was measured at 725 nm using spectrophotometer. The results were expressed as tannic acid equivalents in milligram per gram of the extract, using a standard curve generated with tannic acid and calculation was carried out by using an equation. The equation is given below:
Absorbance = 0.001 × tannic acid (μg) + 0.0033
Results
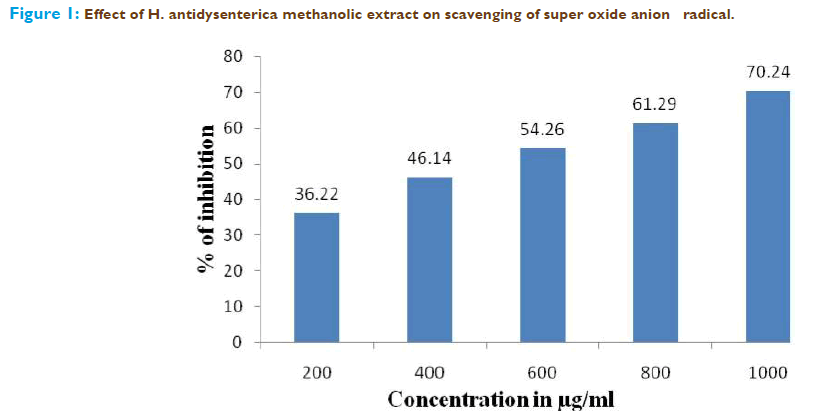
The superoxide anion derived from dissolved oxygen by phenazine methosul- phate/NADH coupling reaction reduces nitro blue tetrazolium. The decrease in the absorbance at 560 nm with the plant extract thus indicates the consumption of superoxide anion in the reaction mixture. As depicted in Figure.1, the methanolic extract possesses the scavenging activity with efficacy of 50% inhibition at 500μg/ml as equivalents to tannic acid.
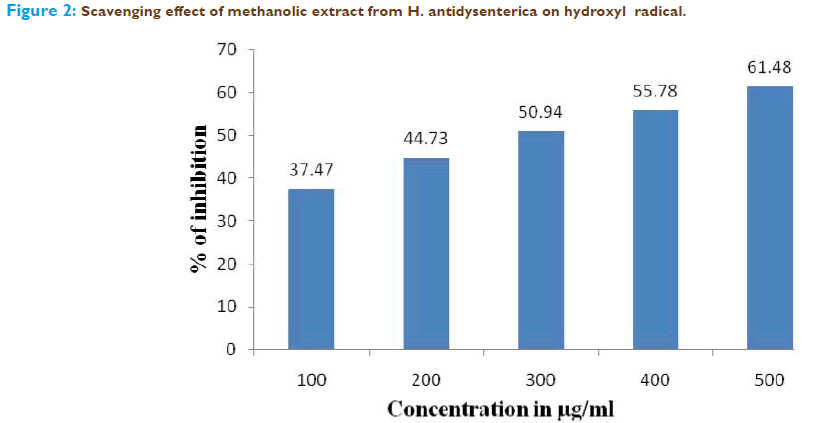
To attack the substrate deoxyribose, hydroxyl radicals were generated by the reaction of Fe3+–EDTA together with H2O2 and ascorbic acid. When the plant extract was incubated with the above reaction mixture, it could prevent the damage against the sugar. The results are shown in Figure.2, the required concentration for 50% inhibition was found to be 285μg/ml as equivalents to tannic acid.
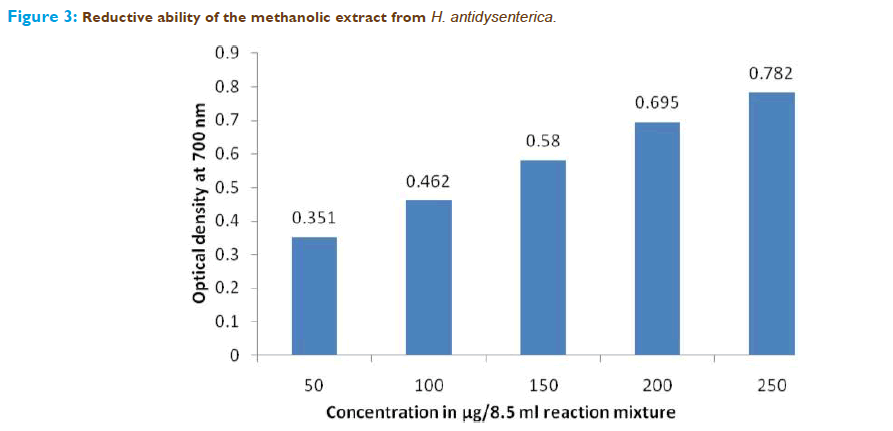
Figure.3 shows the reductive capabilities of the plant extract. The reducing power of the methanolic extract of H.antidysenterica as shown in figure there is a dosedependent increase in absorbance. This indicates that, alcoholic fraction of H.antidysenterica plant extract could reduce the most Fe3+ to Fe2+ions.
The total phenolic content of alcoholic extract of H.antidysenterica was expressed as 41.32 mg of tannic acid equivalents per gram of extract.
Discussion
Free radicals have aroused significant interest among scientists in the past decade. Their broad range of effects in biological systems has drawn the attention of many experimental works. It has been proved that these mechanisms may be important in the pathogenesis of certain diseases and ageing. There are many reports that support the use of antioxidant supplementation in reducing the level of oxidative stress and in slowing or preventing the progress of complications associated with diseases [25]. Many synthetic antioxidant components have shown toxic and/or mutagenic effects, which have shifted the attention towards the naturally occur- ring antioxidants. Numerous plant constituents have proven to show free radical scavenging or antioxidant activity [26]. Flavonoids and other phenolic compounds (hydroxyl cinnamic derivatives, catechines, etc.) of plant origin have been reported as scavengers and inhibitors of lipid peroxidation [27].
In the PMS/NADH-NBT system, superoxide anion derived from dissolved oxygen by PMS/NADH coupling reaction reduced NBT. The decrease of ab- sorbance at 560 nm with antioxidants thus indicates the consumption of superox- ide anion in the reaction mixture. In the present study, the methanolic extract of H. antidysenterica has shown a marked activity and absorbance values shown that there is dose dependent superoxide anion scavenging activity. The extract was ex- amined for its ability to act as OH• scavenging agent. Fe3+–EDTA was incubated with H2O2 and ascorbic acid at pH 7.4; free hydroxyl radicals formed in solu- tion and were detected by their ability to degrade 2-deoxyribose into fragments, upon heating with TBA at low pH form a pink chromogen [20]. While H. antidysenterica extract was added to the reaction mixture the hydroxyl radicals were removed and prevented the degradation of 2-deoxyribose as mentioned above. The phenolic compounds present in the extract may contribute directly to antioxidative action [28]. These results indicate that polyphenols present in extract could be responsible for the beneficial effects. For the measurement of reductive ability, we investigated the Fe3+ to Fe2+ transformation in the presence of methanolic extract. The reducing power of the methanolic extract of H. antidysenterica increases with the increased concentration of the methanolic extract of H. antidysenterica. Compelling evidences indicate that methanolic extract of H. antidysenterica stem bark contain high concentration of phenolic content. Previously reported literature indicated the significance of phenolic compounds in preventing the ROS effect [29-32]. Interestingly in our study we have found higher concentration of phenolic compounds in the tested methanolic extract of H. antidysenterica. This phenolic concentration may be responsible for the antioxidant potency of the extract against different free radicals generated.
Conclusion
This study suggested that the H. antidysenterica plant extract possess antioxidant activity, which might be helpful in preventing or slowing the progress of various oxidative stress- related diseases. Further investigation on the isolation and identification of antioxidant component(s) in the plant may lead to chemical entities with potential for clinical use.
Acknowledgements
The authors are grateful to Dr. H. Manjunatha, Department of PG Studies and Research in Biotechnology, KuvempuUniversity, Karnataka, India for his assistance in carrying out the research work.
References
- Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 1999.
- Yildirim A, Mavi A, Oktay M, et al. Comparison of antioxidant and antimi- crobial activities of tilia (Tilia argenta Desf Ex DC), sage (Salvia triloba L.) and black tea (Camellia sinensis) extracts. Journal of Agricultural and Food Chemistry. 2000; 48: 5030?5034.
- Gulcin I, Oktay MO, Kufrevioglu OI, et al. Determination of antioxidant activity of lichen Cetraria islandica (L.) Ach. Journal of Ethnopharmacol- ogy. 2002b; 79: 325?329.
- Kourounakis AP, Galanakis D, Tsiakitzis K. Synthesis and pharmacological evaluation of novel derivatives of anti-inflammatory drugs with increased antioxidant and anti-inflammatory activities. Drug Development Research. 1999; 47: 9?16.
- Gulcin I, Buyukokuroglu ME, Oktay M, et al. On the in vitro antioxidant properties of melatonin. Journal of Pineal Research. 2002a; 33: 167? 171.
- Sherwin ER. Antioxidants. In: Food Additives. Branen R. (ed.), New York: Marcel Dekker; 1990. p. 139?193.
- Grice HC. Safety evaluation of butylated hydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food and Chemical Toxicology. 1986; 24: 1127?1130.
- Wichi HP. Enhanced tumor development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal aquamous epithelium. Food and Chemical Toxicology. 1988; 26: 717?723.
- Hettiarachchy NS, Glenn KC, Gnanasambandam R, et al. Natural antioxidant extract from fenugreek (Trigonella foenumgraecum) for ground beef patties. Journal Food Science. 1996; 61: 516?519.
- Pryor WA. The antioxidant nutrient and disease prevention ? what do we know and what do we need to find out? American Journal of Clinical Nutri- tion. 1991; 53: 391?393.
- Kinsella JE, Frankel E, German B, et al. Possible mechanism for the protec- tive role of the antioxidant in wine and plant foods. Food Technology. 1993; 47: 58?89.
- Lai LS, Chou ST, Chao WW. Studies on the antioxidative activities of Hsian- tsao (Mesona procumbens Hemsl) leaf gum. Journal of Agricultural and Food Chemistry. 2001; 49: 963?968.
- Gulcin I, Oktay M, K?recci E, et al. Screening of antioxidant and antimicro- bial activities of anise (Pimpinella anisum L.) seed extracts. Food Chemistry. 2003; 83: 371?382.
- Bhattacharjee SK. Handbook of Medicinal Plants. Jaipur, India: Pointer Pub- lishers; 2000. p. 183.
- Guha Bakshi DN, Sensarma P, Pal DC. A Lexicon of Medicinal Plants in India. (Vol II), Calcutta, India: Naya Prokash Publishers; 2001. p. 356-358.
- Chopra RN, Chara IC, Handa KL, et al. Indigenous Drugs of India. New Delhi, India: Academic Press; 1982. p. 342.
- Prajapati ND, Purohit SS, Sharma AK, et al. A Handbook of Medicinal Plants. Jodhpur, India: Agrobios Publishers; 2004. p. 273.
- Sujan Ganapathy PS, Sudeep HV, Padmalatha Rai S, et al. Screening of crude extracts of Holarrhena antidysenterica Wall. against clinically important pathogenic strains. Medicinal and Aromatic Plant Science and Biotechnology. 2008; 2: 68-71.
- Sujan Ganapathy PS, Ramachandra YL, Padmalatha Rai S. Anti-inflam- matory and analgesic activities of Holarrhena antidysenterica Wall. leaf ex- tract in experimental animal models. International Journal of Biomedical and Pharmaceutical Sciences. 2010; 4: 101-103.
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the re- action of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications. 1972; 46: 849-54.
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple ?test tube? assay for determination of rate constants for reaction of hydroxyl radicals. Analytical Biochemistry. 1987; 165: 215?219.
- Ohkawa H, Ohish N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979; 95: 351?358.
- Oyaizu M. Studies on product of browing effect reaction prepared from glucose amine. Japanese Journal of Nutrition. 1986; 44: 307?315.
- Shahidi F, Naczk M. Phenolic compounds in cereals and legumes. In: Food Phenolics: Sources, Chemistry, Effects, Applications. Technomic Publ. Co. Inc., Lancaster; 1995. p. 13?18.
- Rose WM, Creighton MO, Stewart DHPJ, et al. In vivo effects of vitamin E on cataractogenesis in diabetic rats. Canadian Journal of Ophthalmology. 1982; 17: 61?66.
- Aruoma OI, Cuppett SL. Antioxidant Methodology In vivo and In Vitro Con- cepts. AOCS Press, Champaign, Illinois; 1997. p. 41?172.
- Formica JV, Regelson W. Review of the biology of quercetin and related bifla- vonoids. Food and Chemical Toxicology. 1995; 33: 1061?1080.
- Duh PD, Tu YY, Yen GC. Antioxidant activity of aqueous extract of harnjyur (Chyrsanthemum morifolium Ramat). Lebensmittel- Wissenschaft and Tech- nologie. 1999; 32: 269?277.
- Velioglu VS, Mazza G, Gao L, et al. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of Agricultural and Food Chemistry. 1998; 46: 4113?4117.
- Wang M, Shao Y, Li J, et al. Antioxidative phenolic glycosides from sage (Salvia officinalis). Journal of Natural Products. 1999; 62: 454?456.
- Gil MI, Tomas-Barberan FA, Hess PB, et al. Antioxidant activity of pome- granate juice and its relationship with phenolic composition and processing. Journal of Agricultural and Food Chemistry. 2000; 48: 4581?4589.
- Roginsky V, Lissi EA. Review of methods to determine chain-breaking anti- oxidant activity in food. Food Chemistry. 2005; 92: 235?254.