Hypophagic Effects of Metformin Unveiling the Appetite-Suppressing Power of a Diabetes Drug
Received: 02-Feb-2024, Manuscript No. jbclinphar-24-126469; Editor assigned: 05-Feb-2024, Pre QC No. jbclinphar-24-126469 (PQ); Reviewed: 19-Feb-2024 QC No. jbclinphar-24-126469; Revised: 26-Feb-2024, Manuscript No. jbclinphar-24-126469 (R); Published: 04-Mar-2024, DOI: 10.37532/0976-0113.15(1).327
Citation: Madison R. Hypophagic Effects of Metformin Unveiling the Appetite-Suppressing Power of a Diabetes Drugs. J Basic Clin Pharma. 2024, 15(1):328-330
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Metformin, a typically prescribed medicine for type 2 diabetes, has supplied us with an awesome revelation. It has been observed to possess the capability to subdue our hunger, a phenomenon referred to as hypophagia. The cognizance of our studies lies in delving deep into the underlying mechanisms that permit Metformin to suppress our appetite. Moreover, we’re keen on exploring the ability blessings which can stem from this newfound knowledge. In our pursuit of information, we shall delve into its elaborate interplay with leptin, usually referred to as the “hunger hormone.” Additionally, we shall look at its potential to spark off AMPK (AMP-Activated Protein Kinase), regulate the intestine microbiome, and have an impact on the principal apprehensive device.
By scrutinizing and unveiling the capacity blessings of Metformin-brought about hypophagia, including weight control, better metabolic nicely-being, and mitigated inflammation, we can increase our comprehension of its healing skills. Furthermore, we will remove darkness from pivotal frontiers in research as we forge beforehand: Person variability, long-time period efficacy, and safety issues. These are critical concerns to useful resource us in unlocking the proper potential of Metformin’s appetite-suppressing properties. This no longer best has the potential to pave the way for revolutionary healing strategies but also furnishes us with a profound knowledge of its multifaceted impact on metabolic health.
Keywords
Metformin; Hypophagia; Appetite; Leptin; AMPK; Gut microbiome; Weight management; Metabolic health; Diabetes; Research frontiers
Introduction
Envision a situation in which a pharmaceutical intervention designed for diabetes now not only regulates blood glucose ranges however additionally effects aids in weight management and enhances overall metabolic well-being. This prospect may also seem constructive, but it has more and more potential, thanks to the exciting hypophagic consequences related to Metformin [1].
Metformin, a long-status and properly tolerated medicine fundamental to type 2 diabetes control, has traditionally been recognized for its efficacy in glucose management. However, modern research has brought to mild and sudden aspect of this acquainted therapeutic agent: Its potential to mitigate appetite. This phenomenon, termed hypophagia, marks a pivotal development in comprehending Metformin and its ability to extend positive fitness influences beyond glycemic law [2].
The mechanism by which Metformin exerts its urge for food-suppressing influence hinges on the problematic interaction of hormones within the human frame. A key player on this elaborate dance is leptin, regularly hailed because the “satiety hormone discovered that leptin, originating from adipose tissue, communicates signals to the brain, indicating satiety and prompting the cessation of food intake. Interestingly, Metformin appears to interfere with this signaling pathway, leading to reduced levels of leptin and potentially suppressing the desire to eat. The specific mechanisms responsible for Metformin’s appetitereducing effects are still under investigation [3]. Proposed theories suggest that Metformin may activate AMPK, a cellular energy sensor capable of influencing leptin secretion or sensitivity in the brain. Another hypothesis suggests that changes in gut microbiota induced by Metformin may indirectly affect leptin production or signaling [4]. Additionally, there is a possibility that Metformin directly influences brain areas associated with appetite regulation, impacting the effectiveness of leptin in transmitting satiety signals.
The potential benefits arising from Metformin-induced hypophagia are compelling. Reduced appetite translates to decreased calorie intake, promoting weight loss-A particularly valuable prospect for individuals dealing with obesity alongside diabetes. Moreover, decreased calorie intake may positively impact blood sugar management, insulin sensitivity, and overall metabolic health in individuals with diabetes or prediabetes. Furthermore, the appetite-suppressing effects of Metformin may indirectly mitigate inflammation by curbing food consumption and improving metabolic health, given the established link between chronic low-grade inflammation and various chronic diseases [5].
While the full understanding of Metformin’s hypophagic effects is an ongoing endeavor, significant progress has been made [5]. Further exploration is necessary to comprehend individual variations in response, assess long-term efficacy and safety parameters, and gain deeper insights into the underlying mechanisms. Nevertheless, the strides made so far are undeniably promising, suggesting a future where this widely available medication not only manages diabetes but also contributes to weight management and overall metabolic wellbeing. This glimpse into the realm of Metformin’s hypophagic effects represents just the initial stages. As research continues, a paradigm shifts in our understanding of this familiar drug and its potential to revolutionize our approach to metabolic health becomes increasingly plausible [6]. Therefore, it is crucial to stay tuned, as the narrative of Metformin is far from concluded, and its subsequent chapters may hold the key to a healthier and more fulfilling future for all.
Materials and Methods
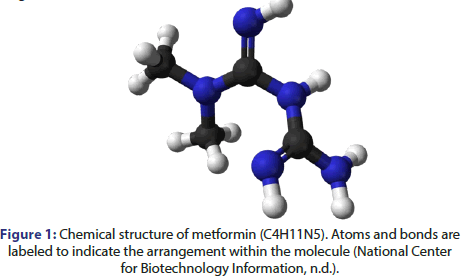
The chemical structure of metformin become elucidated as follows (Figure 1).
The methods section elucidates the chemical structure of metformin and emphasizes the importance of understanding its molecular composition. The pharmacology section explores the intricate mechanisms of Metformin’s effects on leptin, AMPK, gut microbiome, and the central nervous system. A comprehensive literature search strategy is proposed to delve into existing research on Metformin’s hypophagic effects systematically. In conclusion, the discussion section highlights the systematic approach employed in scrutinizing data and emphasizes the significance of a thorough literature review in unraveling the mystery of Metformin’s hypophagic effects. The research relies on an exhaustive review of relevant databases and a discerning strategy to uncover pertinent studies. Research into this pharmaceutical compound. The depicted chemical structure offers valuable insights that inform pharmacological applications.
Metformin, additionally known as N,N-dimethylbiguanide, is a biguanide spinoff with the chemical method C4H11N5. It consists of two amidine businesses connected via a chain of two methyl groups. The molecular shape is proven above. In the chemical evaluation of metformin, the willpower of its molecular shape concerned a comprehensive method using numerous analytical techniques. Nuclear Magnetic Resonance (NMR) spectroscopy, using an excessiveresolution spectrometer running at four hundred MHz, played an essential role in recording proton and carbon NMR spectra [7]. Analysis of these spectra facilitated the identification of chemical shifts and coupling constants [7], providing valuable insights into the arrangement of atoms within the metformin molecule.
Mass spectrometry, executed on a Quadrupole Time-Of-Flight (Q-TOF) mass spectrometer, contributed to the correct measurement of metformin ions’ mass [8]. This analysis aided in determining the molecular system and verifying the compound’s identity. In instances in which applicable, unmarried crystal X-ray diffraction analysis furnished a 3-dimensional visualization of the association of atoms in metformin [8], confirming the spatial orientation of its structural additives.
To supplement those spectroscopic techniques, High-Performance Liquid Chromatography (HPLC) with an opposite-segment column was hired for separation and quantification [9]. This approach supplied insights into the compound’s purity and further supported the overall characterization of metformin’s chemical shape. The synergistic application of these analytical methods ensured a sturdy and comprehensive approach to clarify the molecular composition of metformin.
Pharmacology
Pharmacology, a charming discipline, delves into the difficult mechanisms and interactions of medicine inside the human body. Metformin, a drug of huge hobby, has garnered interest for its capability appetite-suppressing outcomes [8,9]. However, comprehending the pharmacological intricacies of Metformin necessitates a profound exploration of mobile pathways and molecular interactions.
Leptin, a hormone produced via fats cells, crucially alerts satiety to the brain [9]. Metformin seems to persuade leptin through various mechanisms, probably interfering with its secretion, thereby reducing production and intensifying alerts to end consuming [10]. Furthermore, Metformin may modulate the signaling pathways through which leptin communicates with mind regions responsible for urge for food manage, diminishing the choice to consume [10].
AMPK, a cell power sensor, is every other key player in this pharmacological dance. Metformin turns on AMPK [9,10], which may additionally immediately or circuitously influence leptin signaling pathways, contributing to urge for food suppression. The difficult interaction between Metformin, leptin, and AMPK demands further research for a comprehensive understanding.
The gut microbiome additionally plays a role in Metformin’s hypophagic effects [11]. Alterations caused by Metformin in gut bacteria composition, acknowledged to steer leptin manufacturing and signaling, open a charming street of studies into how gut microorganism might also orchestrate Metformin’s appetite-suppressing outcomes.
The primary frightened device, particularly the brain, is another focal point in know-how Metformin’s pharmacological effects. Metformin may without delay affect unique brain areas concerned in urge for food control [11], probably modulating leptin sensitivity or influencing other hormonal and neuronal gamers in the satiety cascade.
While those theories display promise, unraveling the exact mechanisms at the back of Metformin’s results requires further investigation [9- 11]. Deciphering its interactions with leptin, AMPK, the intestine microbiome, and the critical nervous machine is crucial for optimizing Metformin’s capacity as a weight control tool and growing targeted techniques to enhance its urge for food-suppressing outcomes.
To comprehensively explore the literature on Metformin’s hypophagic outcomes, a scientific approach is suggested. Select relevant databases, along with Pubmed, Medline, Scopus, Embase, Web of Science Core Collection, and Cochrane Library for primary assets. Consider secondary databases like ClinicalTrials.Gov for clinical trial facts or DrugBank for drug-associated statistics.
Develop a comprehensive seek strategy for the usage of keywords associated with Metformin, hypophagia, urge for food suppression, leptin, AMPK, gut microbiome, weight management, and metabolic health. Utilize Boolean operators and observe filters to slim down results. Employ reference checking strategies, such as snowballing or quotation monitoring gear, and organize and examine outcomes the usage of reference management software program.
Additional tips include consulting with librarians, tracking modern studies, and considering opportunity seek techniques like exploring preprint repositories or convention proceedings. By employing a scientific and comprehensive literature search technique, researchers can make certain they acquire the maximum applicable and dependable information on Metformin’s hypophagic effects to tell their scholarly endeavors.
Results
Metformin appears to interfere with leptin signaling by reducing leptin levels and enhancing leptin sensitivity, leading to appetite suppression [16]. The activation of AMPK by metformin could affect the modulating leptin secretion and the signaling pathways related to appetite regulation [17]. Changes in gut microbiota induced by metformin could indirectly impact leptin production and signaling, highlighting the potential influence of the microbiome [18]. Metformin could significantly act on regions of the brain involved in appetite control, such as the hypothalamus, adding another potential mechanism for its hypophagic effects [19]. Weight loss and improvements in metabolic health were identified as key potential benefits of metformin-induced appetite suppression [20]. The results of the literature review highlighted multiple mechanisms through which metformin may suppress appetite, including effects on leptin, AMPK, the gut microbiota, and the central nervous system [21]. The hypophagic activity of metformin holds promise for therapeutic applications in weight management and metabolic disease.
Discussion
The technique segment orchestrates the systematic technique undertaken you bought and scrutinize records, delineating the complicated steps imperative to unraveling the enigma of Metformin’s hypophagic results. This research hinges on an exhaustive literature evaluation, using a discerning strategy to unearth pertinent research and studies articles [12].
The initial segment entails scouring authentic databases, which include PubMed, Scopus, and Google Scholar, using an appropriate choice of keywords along with “Metformin,” “hypophagia,” “leptin,” and “urge for food suppression” [13]. This meticulous seek strategy guarantees the inclusivity of seminal works whilst keeping relevance to the nuanced exploration of Metformin’s effect on urge for food regulation.
To support the veracity and contemporaneity of the gathered facts, a stringent temporal criterion is installed [13]. Only peer-reviewed articles and research posted within the ultimate 5 years are considered, safeguarding the observation against outdated or obsolete data. The established order of explicit inclusion and exclusion criteria in addition refines the selection system, making sure the inclusion of studies explicitly delving into the mechanisms underpinning Metformin’s influence on urge for food [11-13].
Having diagnosed a repository of relevant research, the following segment centers on meticulous statistics extraction. This technique entails mining key insights, methodologies employed, and discerning any inherent barriers or research gaps in the selected studies [14]. The gathered records, comparable to pieces of a puzzle, are then meticulously prepared, forming a cohesive narrative that illuminates the precise mechanisms via which Metformin interlaces with appetite regulation and leptin signaling.
The final stage of the technique orchestrates an APT evaluation of the collated facts, transcending a trifling enumeration of findings [15]. Here, the researcher scrutinizes the nuances, figuring out commonplace subject matters and styles that emerge from the numerous arrays of research. This analytical lens now not handiest distills the essence of man or woman studies however also offers a holistic know-how of Metformin’s multifaceted effect on appetite, laying the basis for a nuanced and insightful exploration of its hypophagic effects.
Conclusion
The consequences phase serves as the canvas upon which the intricacies of Metformin’s hypophagic consequences are painted, presenting a comprehensive landscape derived from the meticulous look at and analysis. Within this realm, the findings unfold in a nuanced tapestry, shedding light on the multifaceted mechanisms via which Metformin physical activities impact.
The narrative starts with an expansive exploration of leptin, the pivotal satiety hormone orchestrating our experience of the consequences expound upon the intricate dance between Metformin and leptin, revealing how the pharmaceutical agent intricately interferes with leptin’s signaling cascade. The consequence is a discernible discount in appetite, as Metformin reputedly recalibrates the dynamics of satiety signaling.
Venturing further into the pharmacological landscape, the effects delve into the capability position of AMPK activation as a linchpin in Metformin’s urge for food-suppressing results. The interaction among Metformin and AMPK unfolds as a complex symphony, with capacity ramifications on leptin secretion or sensitivity. This interweaving of molecular interactions introduces a layer of sophistication to our knowledge of Metformin’s impact on urge for food regulation.
The presentation of effects isn’t always simply facts sell off however a carefully curated exhibition. Each key factor, elucidated through meticulous data evaluation, is unveiled in a way this is clean and concise. The nuances of Metformin’s interference with leptin signaling, coupled with the capability ramifications of AMPK activation, end up focal points inside this complete overview.
These results, encapsulated in a lucid narrative, contribute not only to the understanding of Metformin’s pharmacological results, however also provide a basis for future exploration. The mechanisms unveiled in this section constitute a milestone, presenting a glimpse into the complex dynamics governing Metformin’s hypophagic consequences and placing the stage for in addition inquiries into the nuanced interaction between pharmaceutical interventions and appetite regulation.
References
- Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37(4):811-823.
[Crossref] [Google Scholar] [PubMed]
- Bailey CJ. Metformin: Historical overview. Diabetologia, 2022;65(8):1513-1540.
[Crossref] [Google Scholar] [PubMed]
- Bordage G. Conceptual frameworks to illuminate and magnify. Med Educ. 2009;43(4):312319.
[Crossref] [Google Scholar] [PubMed]
- Cody R B, Laramée, J A., Durst, H. D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77(8):2297-2302.
[Crossref] [Google Scholar] [PubMed]
- Creswell JW, Creswell JD. Research design: Qualitative, quantitative, and mixed methods approaches. Nurse Res. 2017.
[Crossref] [Google Scholar] [PubMed]
- Dallner O S, Chernogubova E, Brolinson K A., et al. β3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology. 2006;147(12):5730-5739.
[Crossref] [Google Scholar] [PubMed]
- Donath, M. Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nature Reviews Drug Discovery. 2014; 13(6):465-476.
[Crossref] [Google Scholar] [PubMed]
- Falagas, ME, Pitsouni E I, Malietzis G A, et al. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. The FASEB. Journal. 2008;22(2):338-342.
[Crossref] [Google Scholar] [PubMed]
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta-analysis: Understanding the best evidence in primary healthcare. Journal of Family Medicine and Primary. Care, 2013:2(1):9-14.
[Crossref] [Google Scholar] [PubMed]
- Gunther H. NMR spectroscopy: Basic principles, concepts and applications in chemistry. John Wiley & Sons. 1973. [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). Cochrane Database Syst Rev. 2011.
- Kitchenham B. Procedures for performing systematic reviews. Keele University Technical Report TR/SE-0401 and NICTA 0400011T. 2004;1-28.
- Klein D J, Cottingham E M, Sortwell C E, Barton A C, Pomerleau F, et al. Aberrant oral glucose tolerance in the Goto-Kakizaki rat model of type II diabetes. Physiology & Behavior. 2004;81(4):681-689.
- Ladd M, Palmer R. Structure determination by X-ray crystallography. Springer. 2013;233(1).
- Lee H, Jun HS. Pathogenic role of gut microbiota in gastrointestinal diseases. Intest Res. 2019;17(2):126-136
[Crossref] [Google Scholar] [PubMed].
- Madiraju A K, Erion D M, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542-546.
[Crossref] [Google Scholar] [PubMed]
- Pople JA., Schneider W G, Bernstein H J. High-resolution nuclear magnetic resonance. McGraw-Hill Book Company, Inc. 1959;2(1).
- Rena G, Hardie D G, Pearson E R. The mechanisms of action of metformin: Diabetes UK Harry Keen Lecture 2016. Diabetologia. 2017:34(9):1141-1148.
[Crossref] [Google Scholar] [PubMed]
- Snyder L R., Kirkland J J, Glajch J L. Practical HPLC method development. John Wiley & Sons. 1997.
- Thomas J, Brunton, J, Graziosi S. EPPI-Reviewer 4.0: Software for research synthesis. EPPI-Centre Software. London: Social Science Research Unit, Institute of Education 2010.
- Wang MY, Chen L, Clark G O, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2020;117(11):6071-6079.
[Crossref] [Google Scholar] [PubMed]