Gram Negative Bacterial Sepsis in a Cancer Centre: Bacteriological Spectrum and Antibiotic Susceptibility Profiles
- *Corresponding Author:
- Hemant J Vira
Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, Kharghar, Navi Mumbai-410 210, Maharashtra, India
E-mail: virahemant@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Introduction: Early detection and treatment of sepsis can be lifesaving for critically ill patients. Sepsis caused by Gram negative organisms is on the rise in cancer patients. It is important to identify the infecting organism and test the effectiveness of antibiotics against it as soon as possible. We aimed at studying the in vitro effectiveness of commonly used antibiotic against pathogenic Gram negative organisms in cancer patients with sepsis. Methods: We conducted a four year and one month study of all Gram-negative isolates from blood samples of patients received from oncology units in our hospital. All isolates were processed as per standard microbiology laboratory operating procedures (SOPs). Isolates were identified to species level and susceptibility tests were performed, interpreted and reporting of results was as per Clinical Laboratory Standards Institute (CLSI) guidelines. Results: A total of 5391 blood cultures were received during the study period of which 179 tested positive for Gram negative bacterial sepsis. Escherichia coli Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. were most commonly encountered pathogens. Resistance to piperacillin-tazobactam in the Enterobacteriaceae group was high at 63.46% in K. pneumoniae and 46.4% in E. coli respectively. Although resistance to cefoperazone–sulbactum was 65% in K. pneumoniae, three-fourths of the isolates remained susceptible to ceftazidime. K. pneumoniae and E. coli also showed a very high rate of resistance 69.3% and 83.2% respectively to the fluoroquinolone ciprofloxacin. Half of the Acinetobacter strains were resistant to meropenem a carbapenem antibiotic. Three isolates of Klebsiella pnuemoniae were resistant to Colistin. Conclusion: A high level of resistance to cephalosporins, fluoroquinolones and beta-lactam – beta lacatamase inhibitor combinations is seen in Gram negative sepsis causing organisms in cancer patients, particularly E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Resistance to carbapenems is also on the increase in this group of organisms.
Keywords
Gram negative, sepsis, cancer, antibiotic sensitivity
Introduction
Cancer figures among the leading cause of mortality and morbidity worldwide, with approximately 14 million new cases and 8.2 million cancer related deaths in 2012 according to a report published by WHO world cancer report 2014, with the number of new cases expected to rise by 70% over the next two decades.[1] Cancer patients are immunocompromised due to the disease itself and also due to multiple factors such as chemotherapy, radiotherapy, impairment of normal leukocyte function, and use of corticosteroids. This leads to increased bloodstream infections in cancer patients.[2] In a study conducted on hematopoietic stem cell transplant (HSCT) recipients it was reported that 37% of the patients had Gram negative bacterial sepsis of blood.[2,3] Also Williams et al. reported 606,176 cancer hospitalizations of which severe sepsis was present in 29,795 (4.9%) of the patients.[4] Bloodstream infections due to Gram-negative bacilli (GNB) are common in cancer patients during aggressive immunosuppressive therapy. A study carried out in patients with hematological malignancies and solid neoplasms in Hospitals in the United States by Wisplinghoff et al. reported Gramnegative organisms accounted for 22% and 14% of all blood stream infections (BSIs).[5] In a prospective study conducted in a paediatric hemato-oncology unit of a tertiary care hospital, blood stream infections accounted for half of the total infections.[6] Another Indian study on BSIs showed that common bacterial isolates from patients with cancer were Pseudomonas spp. (30.37%), Staphylococcus aureus (12.6%) and Acinetobacter spp. (11.57%).[7] Similarly in a blood stream infections in pediatric patients at a tertiary care cancer centre in India it was reported that Lactose fermenting Enterobacteriaceae i.e., Escherichia coli (28.4%), Klebsiella pneumoniae (22.1%), and Enterobacter (4.8%) accounted for more than half of all GNB. Pseudomonas spp accounted for 53 (25.5%) and Acinetobacter spp. 19 (9.1%) of GNB.[8]
In recent years due to rampant use of antibiotics and also due to evolution of various resistance mechanisms in Gram negative bacteria there has been widespread increasing resistance.[9] The quinolones of which ciprofloxacin is the most effective against Gram negative bacteria, most importantly Pseudomonas aeruginosa has been reported to show resistance due to gyrA and parC mutations in the gene encoding DNA gyrase enzyme.[10] Also various other mechanisms of quinolone resistance have been elucidated such as over expression of efflux pump system and the innate impermeability of the membrane in Gram negative bacteria.[11] In aminoglycosides, the noted mechanism of resistance include the deactivation of aminoglycosides by aminoglycoside-modifying enzymes which act on specific sites of the aminoglycosides causing acetylation, adenylation or phosphorylation via aminoglycoside phosphotransferase (APH).[12] Amikacin the most successful antibiotic of its class is effective against Gram negative bacterial infection which are found to be resistant to gentamicin and tobramicin, however resistance mechanisms such as mutations in the ribosomal sites essential for its binding has been reported.[13] The extended spectrum beta lactam-beta-lactamase inhibitor (BLBLI) combination piperacillin-tazobactam has been losing effectiveness mainly due a change in the outer membrane protein of the organism that blocks its entry into the periplasmic space.[14] Carbapenem class of antibiotic such as meropenem are often the last line of defence against many Gram negative bacteria that are resistant to other antimicrobial agents.[15] However increasing resistance has been reported throughout the world due to extended spectrum beta-lactamases, mainly New Delhi Metallo-β-lactamase-1 (NDM-1), conferring resistance to all the carbapenems thus raising issues of serious concern in the treatment of Gram negative Enterobacteriaceae blood stream infections in cancer patients.[16,17] In this study we report the antibiotic susceptibility testing (AST) profile of some of the common Gram negative isolates from sepsis cases in cancer patients.
Materials and Methods
A four years and one month study on the effectiveness of the most common antibiotics used against sepsis causing blood stream infections (BSI) Gram-negative isolates obtained from cancer patients was undertaken for the period August 2012 to August 2016. The blood samples were collected and processed for culturing with the relevant protocols of the hospital as per standard microbiology laboratory operating procedures.[18] Briefly blood was aseptically collected in BACTEC 9050 blood culture bottles as per the manufacturer’s recommendations. The samples were then sent to the Microbiology laboratory for further processing. The BACTEC bottles containing the samples were loaded in the BACTEC 9050 system (Becton Dickinson Microbiology Systems, Sparks, MD, USA) and monitored for positive signal for five days. The positive signals when detected were immediately Gram stained and cultured on Blood agar, Chocolate agar and MacConkey agar. The growths obtained were processed as per the standard operating procedures and isolates identified to the species level by means of various biochemical tests and by the Vitek-2 instrument (Biomerieux, France). This was followed with antibiotic susceptibility testing (AST) performed as per Clinical Laboratory Standards Institute (CLSI, USA) guidelines 2012.[19] The four commonly encountered Gram negative isolates Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp were included in the study. The disc diffusion technique was used for antibiotic susceptibility testing. In brief, lawn cultures of appropriate inoculum of respective organisms were performed in Muller Hinton Agar (or Muller – Hinton blood agar for fastidious organisms) and antibiotic discs containing known standard concentration of the respective antibiotics were placed on the surface of the inoculated media and these were than incubated overnight. Zones of inhibition were measured the next day and were correlated with CLSI interpretive breakpoints to characterise them as Sensitive (S), Intermediate (I), and Resistant (R). For Gram-negative, the antibiotics for respective organisms were chosen from the following: Amoxicillinclavulanate (20/10 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), gentamicin (10 μg), amikacin (30 μg), netilmycin (30 μg), cefuroxime (30 μg), cefoltaxime (30 μg), ceftazidime (30 μg), cefepime (30 μg), cefoperazone-sulbactam (75/25 μg), cefepime-tazobactam (30/10 μg), imipenem (10 μg), and meropenem (10 μg). Colistin susceptibility was performed by MIC (minimum inhibitory concentration) method, with E-test strips. Percentages of resistance were calculated for the respective microorganism –antibiotic combinations. Strains of S. aureus ATCC 25923, E. coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853 were used for quality control (QC).
Results
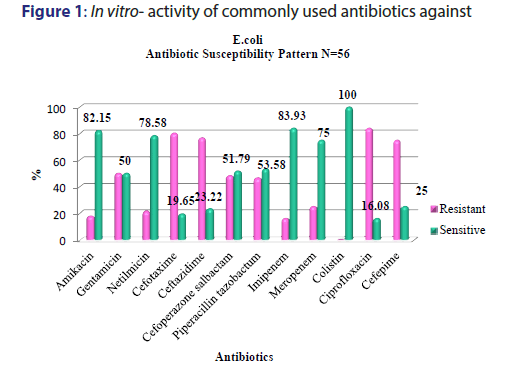
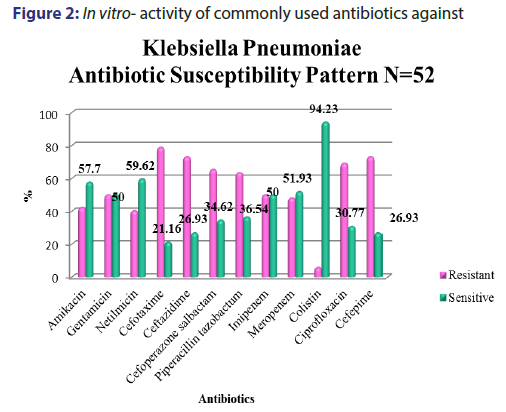
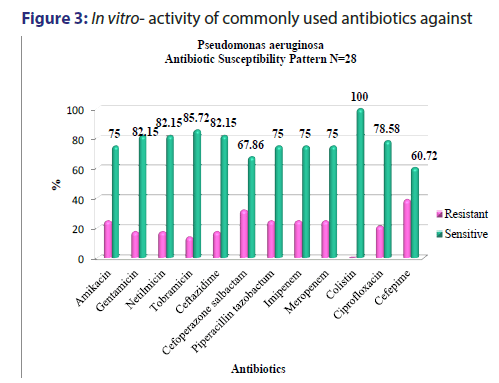
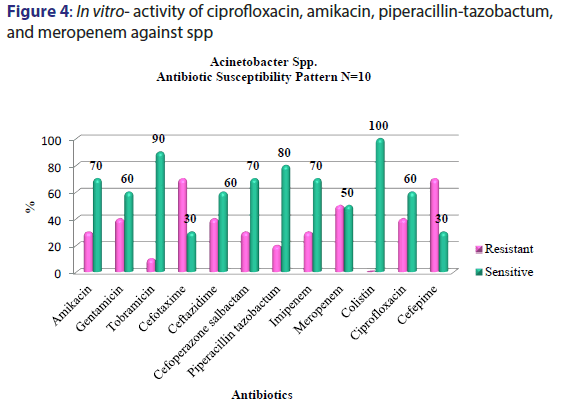
A total of 5391 specimens for blood culture were received during the study period of which 179 were positive for Gram negative bacterial sepsis. Table 1 profiles the Gram negative organisms isolated from the bloodstream of patients. E. coli and K. pneumoniae constituted the majority of Gram negative Enterobacteriaceae blood stream infections (BSI) in our patients accounting for 31.2% and 29.0% of the isolates respectively. This was followed by Pseudomonas aeruginosa (15.6%) and Acinetobacter spp (5.5%) isolates respectively. As depicted in Table 2, most organisms isolated were from HL cancers (105) followed by neurology (19) and gastro-intestinal cancers (15). [Figures 1-4] depicts the activity of amoxicillin-clavulanate, ciprofloxacin, levofloxacin, gentamicin, amikacin, netilmicin, cefuroxime, cefoltaxime, ceftazidime, cefepime, cefoperazone-sulbactam, cefepime-tazobactam, imipenem, meropenem, and colistin against the respective organisms. Ciprofloxacin was effective against 78.58% of P. aeruginosa, whereas resistance was highest in E. coli at 69.23%. Meropenem resistance in K. pneumoniae was high at 48%; in addition colistin resistance was at 5.7%. Piperacillin-tazobactam was effective against 80% of Acinetobacter strains. It can be seen in Figure 2 that 80% of Pseudomonas aeruginosa were susceptible to ceftazidime, while E. coli and Klebsiella pneumoniae were mostly resistant. The resistance to cefoperazone–sulbactum in Klebsiella pneumoniae and E. coli was 65% and 48% respectively.
| Gram negative bacilli | n (%) |
|---|---|
| Escherichia coli | 56 (31.2) |
| Klebsiella pneumoniae | 52 (29.0) |
| Klebsiella oxytoca | 01 (0.5) |
| Aeromonas hydrophila | 01 (0.5) |
| Pseudomonas fluroscence | 02 (1.1) |
| Pseudomonas aeruginosa | 28 (15.6) |
| Pseudomonas stutzeri | 03 (1.6) |
| Shewanella putrefaciens | 01 (0.5) |
| Enterobacter cloacae | 02 (1.1) |
| Acinetobacter spp | 10 (5.5) |
| Stenotrophomonas maltophilia | 03 (1.6) |
| Salmonella spp | 08 (4.4) |
| Chryseobacterium indologenes | 02 (1.1) |
| Roseomonas gilardii | 01 (0.5) |
| Pantoea spp. | 02 (1.1) |
| Brevundimonas diminuta | 02 (1.1) |
| Moraxella spp. | 02 (1.1) |
| Rhizobium radiobacter | 01 (0.5) |
| Morganella morganii | 02 (1.1) |
| Total (n) | 179 |
Table 1: Gram negative pathogens isolated in cancer patients with sepsis
| Cancer Disease Organism | Hemato- Lymphoid n (%) |
Bone- Soft Tissue n (%) |
Breast n (%) |
Gastrointestinal n (%) |
Gynecology n (%) |
Head & Neck n (%) |
Neurology n (%) |
Paediatric Solid Tumor n (%) |
Thoracic n (%) |
Urology
n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Escherichiae coli | 25 (23.81) |
1 (33.33) |
2 (40) |
7 (46.67) |
7 (70) |
10 (52.63) |
2 (25) |
1 (20) |
1 (20) |
|
| Klebsiella pneumoniae | 38 (36.20) |
1 (20) |
2 (13.33) |
3 (30) |
2 (10.53) |
1 (12.5) |
3 (60) |
2 (40) |
||
| Klebsiella oxytoca | 1 (0.95) |
|||||||||
| Acinetobacter spp. | 4 (3.82) |
2 (13.33) |
1 (33.33) |
1 (5.26) |
2 (25) |
|||||
| Enterobacter cloacae | 1 (0.95) |
1 (33.33) |
||||||||
| Morganella morganii | 1 (0.95) |
|||||||||
| Pseudomonas aeruginosa | 16 (15.25) |
1 (33.33) |
1 (20) |
1 (6.67) |
5 (26.32) |
3 (37.5) |
1 (20) |
|||
| Pseudomonas stutzeri | 2 (1.90) |
1 (5.26) |
||||||||
| Pseudomonas fluroscence | 2 (1.90) |
|||||||||
| Salmonella spp. | 8 (7.62) |
|||||||||
| Pantoea spp. | 2 (1.90) |
|||||||||
| Stenotrophomonas.maltophilia | 1 (0.95) |
2 (13.33) |
||||||||
| Moraxella spp. | 2 (1.90) |
|||||||||
| Shewanella spp. | 1 (20) |
|||||||||
| Roseomonas gilardii | 1 (20) |
|||||||||
| Chryseobacterium indologenes | 1 (0.95) |
1 (33.33) |
||||||||
| Brevundimonas diminuta | 1 (6.67) |
1 (33.33) |
||||||||
| Rhizobium radiobacter | 1 (20) |
|||||||||
| Aeromonas hydrophila | 1 (0.95) |
|||||||||
| Total | 105 (100) |
3 (100) |
5 (100) |
15 (100) |
10 (100) |
3 (100) |
19 (100) |
8 (100) |
5 (100) |
5 (100) |
Table 2: Profile of Gram negative isolates from different types of cancer
Discussion
Septicaemia is a common infection seen in cancer patients.[2,3] This study reports the effectiveness of the commonly used antibiotics against Gram negative bacilli isolated from blood stream infections of cancer patients in our setting. While bacteria (Gram positive and Gram negative) and fungi can cause blood stream infections, recent studies have reported an increase in multidrug resistant (MDR) Gram negative blood stream infections in cancer patients showing resistance to cephalosporins, quinolones, aminogylcosides, penicillin/ beta-lactamase inhibitor combinations, and carbapenems, which is a cause for concern.[9,5] There are variable reports of resistance rates of Gram negative bacilli to the above antibiotics in literature. A study conducted in oncology patients (n=179) in United States reported a 14.6% resistance rate to piperacillin-tazobactam combination among Gram negative organisms in general.[20] We report a resistance of about 41.8% (n=75) to piperacillin-tazobactam combination which is higher than the above mentioned study. The most resistant Gram negative organism to piperacillin-tazobactam (63.46%) in our setting was Klebsiella pneumonia [Figure 2]. E coli resistance to piperacillintazobactam was also high at 46.4%, representing increased resistance rates of these organisms to BLBLI combinations. Increased resistance to ciprofloxacin was reported in a study conducted in a cancer patient population of China which noted Escherichia coli isolates having higher resistance (46%) in bacteremic patients which was in contrast to the high sensitivity to ciprofloxacin in bacteremic patients in western countries.[11] Our results showed very high levels of resistance to ciprofloxacin amongst E. coli and K. pneumoniae isolates (83.92% and 69.3% respectively) [Figure 1]. About half of the Acinetobacter spp. isolates were resistant to carbapenems such as meropenem [Figure 4]. These were multidrug resistant Acinetobacter strains that were resistant to most antibiotics. One study from Uttar Pradesh, India carried out in a tertiary care hospital reported a resistance rate of 28% in Acinetobacter spp. which is less than the findings of our study.[21]
The resistance to the carbapenem meropenem in the Enterobacteriaceae group of organisms particularly E. coliand K. pneumoniae is also a matter of concern. One study reported a carbapenem resistance rates of 6.6% due to carbapenemase production such as KPC-2, IMP-4, and NDM-1 in the Enterobacteriaceae isolates.[22] The molecular studies to characterise the carbapenemase markers were not performed in our setting and as a result it remained uncharacterised. Studies have suggested a high rate of resistance of Pseudomonas to piperacillin-tazobactum, amikacins, and carbapenems.[23,24] However, most Pseudomans aeruginosa isolates from our patients remained susceptible to ciprofloxacin, amikacins, and meropenem [Figure 3]. Although Pseudomonas stutzeri is generally taken to be a contaminant, it has been reported to be a pathogen in immunosuppressed patients and must be correlated clinically in such a setting.[25] Susceptibility to ceftazidime in Pseudomonas aeruginosa was greater than 80% which is in accordance to the study conducted by Micek.[26] Evolving resistance to colistin (5.7%) among the MDRGNBs in our setting is a cause for concern because of limited options. The problem of infection with multidrug resistant organisms is further compounded by the immunocompromised status of cancer patients due to the use of chemotherapy, immunosuppression, catheterization and sub optimal nutrition. The evolution of resistance among Gram negative organisms to the beta- lactams, BLBLIs, fluroquinolones, and recently the carbapenem group of drugs is a major problem facing countries like India. This is particularly true in the case of members of the Enterobacteriaceae group such as Escherichia coli and Klebsiella pneumoniae and organisms such as Pseudomonas aeruginosa and Acinetobacter spp. in the Indian setting. This is attributable to misuse of antibiotics by both health care practitioners and patients and veterinary use of antibiotics. This represents a major cause of concern as it leaves us with few options of effective antibiotics against these MDR organisms. In such cases clinicians are left with no option but to revert to old world drugs such as colistin in treating these infections. In fact, colistin is already being used with increasing frequency in many tertiary care hospitals caring for patients with MDR infections. Fortunately, more than half of E. coli, K. pneumoniae, P. aeruginosa, and Acinetobacter spp. isolates in our study retained clinically useful susceptibility to the aminoglycoside, amikacin which still remains a useful treatment option in our setting. The problem of antibiotic resistance needs to be tackled on a war footing. National level committees are formulating guidelines on antibiotic usage and stewardship to minimize the menace of antibiotic resistance. These include strategies such as restricting over the counter sale of antibiotics, In-hospital antibiotic monitoring and antibiotic policy, stepping up infection control measures, rationalizing antibiotic usage in veterinary practice, enhancing clinical research on ne27wer molecules and developing a national antibiotic monitoring network.[27] It is important for hospitals to regularly monitor resistance trends to the commonly used antibiotics in their setting and implement antibiotic policies and stewardship to contain the continuing threat of antibiotic resistance.
Conclusion
Multidrug resistant Gram negative pathogens are an important cause of blood stream infections causing sepsis in cancer patients. The most common organism isolated was E. coli followed by K. pneumonie, P. aeruginosa and Acinietobacter. The extent of resistance to cephalosporins, quinolones and BLBLIs among Gram-negative bacilli such as E. coli, K. pneumoniae and Acinetobacter spp. is high. There is also a increasing level of antibiotic resistance to the carbapenem goup of drugs particularly in K. pneumoniae and Acinetobacter spp. Adapting selective antimicrobial use of various pharmacological classes based on local epidemiologic data may play an important role in containing this menace of antb ibiotic resistance.
Financial support and sponsorship
Nil.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Stewart BW, Wild CP. World Cancer Report 2014.
- Staudinger T, Hospital C, Publique A. Current insights into severe sepsis in cancer patients. Rev Bras Ter Intensiva 2014;26:335-8.
- Oliveira AL, Souza MD, Carvalho-dias VMH. Epidemiology of bacteremia and factors associated with multi-drug- resistant Gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2007;775-81.
- Williams MD, Braun LA, Cooper LM. Hospitalized cancer patients with severe sepsis : analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8.
- Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current Trends in the Epidemiology of Nosocomial Bloodstream Infections in Patients with Hematological Malignancies and Solid Neoplasms in Hospitals in the United States. Clin Infect Dis 2003;1103-10.
- Gupta A, Kapil A, Kabra SK. Prospective study estimating healthcare associated infections in a paediatric hemato-oncology unit of a tertiary care hospital in north India. Indian J Med Res 2013;138:944-9.
- Prabhash K, Medhekar A, Ghadyalpatil N. Blood stream infections in cancer patients: A single center experience of isolates and sensitivity pattern. Indian J Cancer 2010;47:185-88.
- Thacker N, Pereira N, Banavali SD. Epidemiology of blood stream infections in pediatric patients at a tertiary care cancer centre. Indian J Cancer. 2014;51:438-41.
- Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol 2014;18:56-60.
- Nouri R, Ahangarzadeh M, Hasani A. The role of gyrA and parC mutations in fluoroquinolones-resistant Pseudomonas aeruginosa isolates from Iran. Brazilian J Microbiol 2016;47:925-30.
- Zhang S, Wang Q, Ling Y, Hu X. Fluoroquinolone resistance in bacteremic and low risk febrile neutropenic patients with cancer. BMC Cancer 2015;1-5.
- Gad GF, Mohamed HA, Ashour HM. Aminoglycoside Resistance Rates , Phenotypes , and Mechanisms of Gram-Negative Bacteria from Infected Patients in Upper Egypt. PLoS One 2011;6:1-7.
- Kim J, Park Y, Kwon HJ, Han K, Kang MW. Occurrence and mechanisms of amikacin resistance and its association with b -lactamases in Pseudomonas aeruginosa : a Korean nationwide study. J Antimicrob Chemother 2008;479-83.
- Rice LB, Carias LL, Hujer AM. High-Level Expression of Chromosomally Encoded SHV-1 -Lactamase and an Outer Membrane Protein Change Confer Resistance to Ceftazidime and Piperacillin- Tazobactam in a Clinical Isolate of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2000;44:362-7.
- Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011;37:415-9.
- Walsh TR. Emerging carbapenemases: A global perspective. Int J Antimicrob Agents. 2010;36:S8-S14.
- Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R. New Delhi Metallo-beta lactamase (NDM-1) in Enterobacteriaceae: treatment options with carbapenems compromised. J Assoc Physicians India. 2010;58:147-49.
- Hall GS. Bailey & Scott’s Diagnostic Microbiology, 13. Lab Med 2013;44:e138-e39.
- Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty Second Informational Supplement M100-S22. Wayne, PA: Clinical Laboratory Standards Institute; 2012.
- Marini BL, Hough SM, Gregg KS, Abu-seir H, Nagel JL. Risk factors for piperacillin / tazobactam-resistant Gram-negative infection in hematology / oncology patients with febrile neutropenia. Support Care Cancer 2015;23:2287-95.
- Patel SS, Bhawna B, Kushawaha DS, Sharma RK, Srivastava V, Prasad J. Antibiogram of Acinetobacter Species Isolated from Various Clinical Specimens in a Tertiary Care Hospital. Ann Int Med Den 2016;2:284-7.
- Zhao ZC, Xu XH, Liu MB, Wu J, Lin J, Li B. Fecal carriage of carbapenem-resistant Enterobacteriaceae in a Chinese university hospital. Am J Infect Control. 2014;42:e61-e64.
- Shanthi M, Sekar U, Kamalanathan A, Sekar B. Detection of New Delhi metallo beta lactamase-1 (NDM-1) carbapenemase in Pseudomonas aeruginosa in a single centre in southern India. Indian J Med Res 2014;140:546-50.
- Road E, Harcourt P, State R. Plasmid Profile Analysis of Multidrug Resistant Pseudomonas aeruginosa Isolated from Wound Infections in South West , Nigeria. World Appl Sci J 2012;20:766-75.
- Bisharat N, Gorlachev T, Keness Y. 10-Years Hospital Experience in Pseudomonas stutzeri and Literature Review. The Open Infectious Diseases Journal 2012;6:21-4.
- Micek ST, Lloyd AE, Ritchie DJ. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob. Agents Chemother. 2005;49:1306-11.
- Team C. Chennai Declaration: 5 year plan to tackle the challenge of antimicrobial resistance. Indian J Med Microbiol. 2014;32:221-8.