Evaluation of the antihypertensive activity and alpha adrenergic receptor interaction of cleistanthins A and B
- *Corresponding Author:
- Dr. Ramasamy Raveendran,
Department of Pharmacology, JIPMER, Gorimedu, Puducherry, India.
E-mail: dr.ravee@gmail.com
Abstract
Hypertension was induced in male Sprague Dawley rats with twice weekly administration of deoxycorticosterone acetate (DOCA) salt (20 mg/kg s.c) for 4 weeks. They were divided into eight groups of six animals each viz., hypertensive control, standard (prazosin 1 mg/kg), cleistanthin A 12.5, 25, 50 mg/kg and cleistanthin B 12.5, 25 mg/kg, and 50 mg/kg. One more group served as normal control. The hypertension was induced in 4 weeks, and the animals were given assigned treatment in 5th week. The alteration in blood pressure (BP) was recorded weekly using a rodent noninvasive blood pressure system. At the end of the experiment alpha‑adrenergic receptor response of drugs like adrenaline, nor adrenaline, dopamine (doses 1 µg and 2 µg) was recorded invasively. Two‑way repeated measures ANOVA followed by Bonferroni post‑hoc test was used to analyze the data. The systolic BP and diastolic BP of test groups rose to a higher level after DOCA administration and fell to the normal range (P < 0.05) following the administration of cleistanthins A and B. There were no differences in the weekly heart rate among the groups. In the test group animals pretreated with prazosin and cleistanthins, adrenaline, noradrenaline and dopamine failed to raise the mean arterial pressure and the end‑diastolic pressure from baseline (P > 0.05) cleistanthins A and B exert a significant antihypertensive effect through alpha‑adrenergic receptor blockade similar to prazosin.
Keywords
Alpha blocker, cleistanthin, deoxycorticostrerone acetate, hypertension
Introduction
Cleistanthins A and B are derived from the plant oduvan, Cleistanthus collinus, (Roxb.) Benth. ex Hook.f. a toxic plant which belongs to the family Euphorbiaceae.[1] The plant is widely distributed in southern, central part of India, and South-East Asia. It is consumed as a poison in the form of decoction and paste.[1,2] The patients who consumed the plant developed uncontrolled hypotension, arrhythmias, acute renal failure and electrocardiogram (ECG) changes.[3‑6] The other active principles in C. collinus leaves include diphyllin, collinusin and all the compounds are arylnaphthalene lignin lactones.[7‑13] More than 25 compounds are derived from the plant, but these two compounds are reported to be the major compounds. The compounds are evaluated for pharmacological activities like diuretic activity, anticancer activity, neuromuscular blocking property.[14‑20]
The structure‑activity relationship prediction showed that cleistanthins A and B exert hypotensive activity. [21] The hypotensive effect of cleistanthins A and B was demonstrated by Parasuraman et al. and it was reported that the compounds reduce the blood pressure (BP) on direct intravenous (i.v.) administration in rat invasive models.[22] Kumar et al. demonstrated the alpha‑adrenergic receptor blocking property of C. collinus leaf extract on isolated tissues guinea pig vas deferens and aortic strip.[23] Parasuraman and Raveendran reported that cleistanthins A and B had an alpha‑adrenergic receptor blocking property in isolated guinea pig vas deferens.[24] It was suggested that cleistanthins A and B reduce BP, and it could be due to their alpha‑adrenergic receptor blocking property.[22] The effect of cleistanthins, A and B on BP in repeated doses, was not studied, and their antihypertensive effect was not evaluated using animal models of hypertension. It would be interesting to study whether these compounds act as antihypertensive agents, that is, reduce the BP in rat models of hypertension and if so, whether the antihypertensive effect induced by repeated administration of these compounds is due to the alpha‑adrenergic receptor blockade by these compounds. Hence the present study was planned to evaluate the antihypertensive activity of cleistanthins A and B on deoxycorticosterone acetate (DOCA) salt induced hypertension in rats.
Materials and Methods
The study was approved by the Institutional Animal Ethics Committee. Healthy, adult, Sprague Dawley rats weighing 175-225 g were used for the study. The animals were housed in large, spacious, hygienic cages during the course of the experimental period. The animals were maintained under standard laboratory conditions, and they had 12 h day/night cycle with temperature (24-28°C).The standard rodent pellet feed supplied by Amrut laboratory animal feeds, Sangli (India) was used to feed them and water ad libitum. The animals were fasted for 12 h prior to the experiment with free access to only water.
Preparation of cleistanthins A and B
Cleistanthins A and B were insoluble in water and hence prepared as the suspension with 0.5% carboxy methyl cellulose (CMC) for oral administration in vivo and ex vivo experiments.
Procedure
The animals were allowed to adapt the laboratory conditions for a week. One day prior to the experiment the animals were divided into nine different groups each containing six animals. Among the nine groups, one group served as a normal control. Hypertension was induced in other eight groups with twice weekly administration of DOCA salt (20 mg/kg s.c) for 4 weeks.[25] The alterations in the BP was recorded every week by rodent noninvasive blood pressure (tail cuff method) system. Hypertension was induced in 4 weeks, and when BP reached >140 mmHg, each group was assigned a specific treatment for 7 days in the 5th week. The groups include hypertensive control, standard treatment group (prazosin 1 mg/kg), cleistanthin A at doses of 12.5 mg/kg, 25 mg/kg, 50 mg/kg and cleistanthin B at doses of 12.5 mg/kg, 25 mg/kg, and 50 mg/kg. The LD50 doses of cleistanthins A and B are 1200 mg/kg and 1000 mg/kg respectively. The dose was calculated as per the Organization for Economic Co‑operation and Development guidelines 420 and 421. The dose selected for pharmacological evaluation is <1/10 of the toxic dose based on the dose determination.[14] The hypertensive control group was treated with the vehicle that is, CMC. At the end of the 5th week again the BP was measured noninvasively to monitor the BP change in response to the test drug.
At the end of the experiment invasive BP will be recorded to study the mechanism of action of cleistanthins A and B. The fasted male rats were anaesthetized using urethane (1200 mg/kg).[26] The femoral vein, trachea and carotid artery was cannulated. The femoral vein was used for administering the drugs, and the carotid cannulation was connected to a pressure transducer (data acquisition system AD instruments) for monitoring the changes in BP. The drugs used were adrenaline, nor adrenaline and dopamine at doses of 1 and 2 µg and the BP changes were monitored through carotid artery. The electrodes in the data acquisition system include positive, negative, reference electrodes, and they were connected to left foreleg, right foreleg and left thigh respectively.[27]
Statistical analysis
The results were presented as mean ± standard error of the mean. The statistical test used was two‑way repeated measures ANOVA, followed by Bonferroni post‑hoc test. The statistical test was done using SPSS software version 16 (SPSS Inc. Chicago). P <0.05 was considered as significant.
Results
Noninvasive blood pressure
The three parameters measured using a noninvasive method include systolic BP, diastolic BP, and heart rate and the data on the parameters are presented in Tables 1‑3.
| Group | 0 week(mmHg) | 1-week DOCA(mmHg) | 2 weeksDOCA (mmHg) | 3 weeksDOCA (mmHg) | 4 weeksDOCA (mmHg) | 5 weeks testdrug (mmHg) |
|---|---|---|---|---|---|---|
| Normal control | 105.1 ± 9.7 | 124.3 ± 7.4 | 117.1 ± 6.2 | 101.4 ± 10.2 | 120.3 ± 5.1 | 118.5 ± 3.1 |
| Hypertensive control | 121 ± 3.1 | 132.6 ± 2.3 | 147.8 ± 6.4 | 159 ± 4 | 180 ± 3.9b | 164.8 ± 7.9 |
| Standard (prazosin 1 mg/kg) | 108.1 ± 5.4 | 112.8 ± 3.7 | 130.3 ± 1.7 | 147.9 ± 3.0 | 160.9 ± 2.8b | 103.9 ± 7.9a |
| Cleistanthin A 12.5 mg/kg | 107.8 ± 2.5 | 120.8 ± 10.0 | 128.9 ± 10.9 | 145.1 ± 4.0 | 157.1 ± 5.4b | 120.1 ± 9.9a |
| Cleistanthin A 25 mg/kg | 103.75 ± 5.8 | 123 ± 5.8 | 109.7 ± 4.3 | 130.6 ± 2.3 | 148.7 ± 3.4b | 120.1 ± 24.1a |
| Cleistanthin A 50 mg/kg | 102.5 ± 5.0 | 113.2 ± 2.8 | 119.6 ± 3.6 | 138.7 ± 3.1 | 161.5 ± 1.9b | 123.3 ± 5.1a |
| Cleistanthin B 12.5 mg/kg | 107.3 ± 10.8 | 112.2 ± 6.4 | 128.0 ± 4.1 | 139.6 ± 2.3 | 159.8 ± 3.4b | 124.6 ± 7.3a |
| Cleistanthin B 25 mg/kg | 106 ± 6.1 | 102.1 ± 5.3 | 119 ± 3.0 | 131.6 ± 1.1 | 149.3 ± 2.6b | 123.1 ± 5.9a |
| Cleistanthin B 50 mg/kg | 106.3 ± 2.8 | 112.1 ± 4.3 | 123.1 ± 4.9 | 151.2 ± 2.9 | 170.8 ± 4.5b | 136.3 ± 1.4a |
Values are mean ± SEM. n=6 in each group. aP<0.05 versus DOCA 4 weeks group, bP<0.05 versus baseline value. Two-way repeated measures ANOVA followed by Bonferroni post-hoc test. DOCA: Deoxycorticosterone acetate salt (20 mg/kg s.c), BP: Blood pressure, SEM: Standard error of the mean
Table 1: Effect of cleistanthins A and B on the systolic BP in DOCA rats (noninvasive BP).
| Group | 0 week(mmHg) | 1-week DOCA(mmHg) | 2 weeksDOCA (mmHg) | 3 weeksDOCA (mmHg) | 4 weeksDOCA (mmHg) | 5 weeks testdrug (mmHg) |
|---|---|---|---|---|---|---|
| Normal control | 53.1 ± 4.4 | 58.0 ± 5.7 | 58.1 ± 3.1 | 54.3 ± 2.8 | 69.4 ± 9.7 | 65 ± 3.0 |
| Hypertensive control | 60.6 ± 1.6 | 52.1 ± 5.6 | 64.5 ± 6.0 | 61.3 ± 6.9 | 79 ± 6.1b | 81.7 ± 3.5 |
| Standard (prazosin 1 mg/kg) | 54.8 ± 3.0 | 56.5 ± 1.9 | 62.6 ± 2.2 | 69 ± 1.7 | 80.5 ± 1.4b | 52 ± 4.0a |
| Cleistanthin A 12.5 mg/kg | 57.2 ± 7.9 | 64.5 ± 4.3 | 68.8 ± 3.2 | 70.2 ± 5.7 | 78.3 ± 2.4b | 60 ± 4.97a |
| Cleistanthin A 25 mg/kg | 51.8 ± 6.9 | 50.8 ± 6.8 | 54.8 ± 5.3 | 65.3 ± 1.2 | 74.5 ± 1.8b | 60 ± 4.97a |
| Cleistanthin A 50 mg/kg | 51.6 ± 2.2 | 56.8 ± 1.4 | 59.5 ± 1.8 | 69.2 ± 1.5 | 78.6 ± 2.5b | 61.5 ± 2.67a |
| Cleistanthin B 12.5 mg/kg | 58.2 ± 9.1 | 56 ± 1.3 | 64 ± 0.8 | 69.8 ± 1.1 | 75.7 ± 5.0b | 63.1 ± 3.3a |
| Cleistanthin B 25 mg/kg | 52.8 ± 3.0 | 51.3 ± 2.8 | 59.5 ± 1.4 | 65.8 ± 0.5 | 73.5 ± 1.1b | 61.5 ± 3.0a |
| Cleistanthin B 50 mg/kg | 53.5 ± 2.1 | 55.8 ± 2.2 | 55.3 ± 2.4 | 75.5 ± 1.3 | 84.8 ± 2.3b | 68 ± 0.8a |
Values are mean ± SEM. n=6 in each group. bP<0.05 versus DOCA 4 weeks group,bP<0.05 versus baseline value. Two-way repeated measures ANOVA followed by Bonferroni post-hoc test. DOCA: Deoxycorticosterone acetate salt (20 mg/kg s.c), BP: Blood pressure, SEM: Standard error of the mean
Table 2: Effect of cleistanthins A and B on the diastolic BP in DOCA rats (noninvasive BP).
| Group | 0 week (beats/min) | 1-week DOCA (beats/min) | 2 weeks DOCA (beats/min) | 3 weeks DOCA (beats/min) | 4 weeks DOCA (beats/min) | 5 weeks test drug (beats/min) |
|---|---|---|---|---|---|---|
| Normal control | 341.8 ± 20.8 | 337.6 ± 51.4 | 369.1 ± 41.2 | 302.8 ± 37.8 | 412 ± 34.7 | 445.8 ± 39.1 |
| Hypertensive control | 345.4 ± 45 | 250 ± 58.2 | 264.3 ± 43 | 333.2 ± 61.6 | 398.4 ± 78.4 | 385.8 ± 80.7 |
| Standard (prazosin 1 mg/kg) | 302.6 ± 30.5 | 298.9 ± 22.9 | 301 ± 41.4 | 299.1 ± 40.2 | 382.5 ± 44.1 | 302.4 ± 45.1 |
| Cleistanthin A 12.5 mg/kg | 338.1 ± 64.2 | 251 ± 45.66 | 288 ± 45.8 | 322.6 ± 46.0 | 286.8 ± 36.9 | 201.5 ± 8.9 |
| Cleistanthin B 12.5 mg/kg | 357.5 ± 52.1 | 357.8 ± 47.0 | 328.1 ± 54.2 | 358.6 ± 58.0 | 270 ± 63.2 | 294.8 ± 19.2 |
| Cleistanthin A 25 mg/kg | 337.3 ± 52 | 371.2 ± 46.4 | 331.1 ± 24.9 | 318.8 ± 56.1 | 328.6 ± 40.1 | 305.6 ± 39.5 |
| Cleistanthin B 25 mg/kg | 315.3 ± 35.0 | 311.1 ± 31.4 | 335.2 ± 34 | 386.6 ± 53.4 | 348.8 ± 35.6 | 372.1 ± 35.3 |
| Cleistanthin A 50 mg/kg | 298.8 ± 87.8 | 360.8 ± 86.7 | 264.4 ± 88.7 | 386 ± 24.6 | 339.8 ± 42.4 | 230.4 ± 27.9 |
| Cleistanthin B 50 mg/kg | 282.5 ± 63.5 | 391.3 ± 42.9 | 236.3 ± 34.3 | 225 ± 28.8 | 282.3 ± 21 | 380 ± 61.3 |
Values are mean ± SEM. n=6 in each group. Two-way repeated measures ANOVA followed by Bonferroni post-hoc test. DOCA: Deoxycorticostrerone acetate salt (20 mg/kg s.c), BP: Blood pressure, SEM: Standard error of the mean
Table 3: Effect of cleistanthins A and B on the heart rate in DOCA rats (noninvasive BP).
Systolic and diastolic blood pressure
The baseline values of systolic and diastolic BP of all the groups were in the normal range [Tables 1 and 2]. The systolic and diastolic BP started rising actively from the 3rd week and almost by 4th week the hypertensive control, standard and test groups (cleistanthin treated groups) showed high systolic and diastolic pressure (P < 0.05). The normal and the hypertensive control groups which received the vehicle did not show any significant change in BP (both systolic and diastolic) in the 5th week (P > 0.05). The standard group which received prazosin and the cleistanthin groups showed a significant BP reduction in the systolic and diastolic pressure at the end of the 5th week (P < 0.05).
Heart rate
Table 3 shows that there were no significant alterations in the heart rate in all the groups throughout 5 weeks (P > 0.05).
Invasive blood pressure
The data on mean arterial pressure and end‑diastolic pressure are presented in Figures 1 and 2.
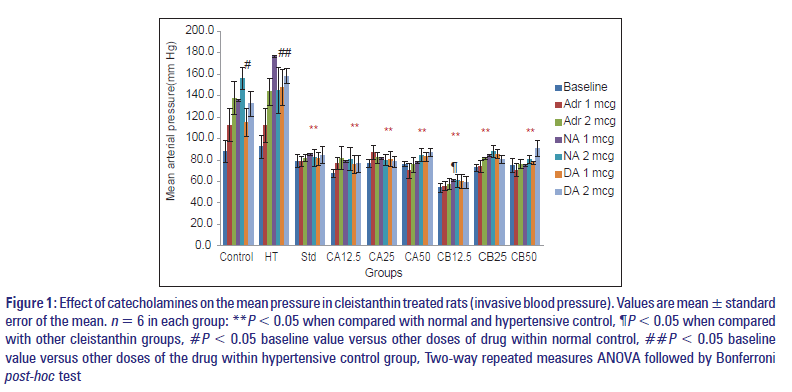
Figure 1: Effect of catecholamines on the mean pressure in cleistanthin treated rats (invasive blood pressure). Values are mean � standard error of the mean. n = 6 in each group: **P < 0.05 when compared with normal and hypertensive control, ¶P < 0.05 when compared with other cleistanthin groups, #P < 0.05 baseline value versus other doses of drug within normal control, ##P < 0.05 baseline value versus other doses of the drug within hypertensive control group, Two-way repeated measures ANOVA followed by Bonferroni post-hoc test
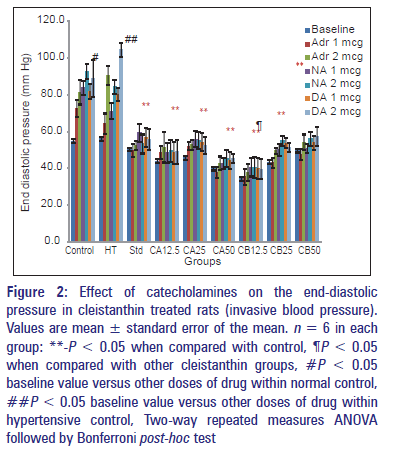
Figure 2: Effect of catecholamines on the end-diastolic pressure in cleistanthin treated rats (invasive blood pressure). Values are mean � standard error of the mean. n = 6 in each group: **-P < 0.05 when compared with control, ¶P < 0.05 when compared with other cleistanthin groups, #P < 0.05 baseline value versus other doses of drug within normal control, ##P < 0.05 baseline value versus other doses of drug within hypertensive control, Two-way repeated measures ANOVA followed by Bonferroni post-hoc test
Mean arterial pressure
The effect of catecholamines on the mean arterial pressure in cleistanthin treated rats was shown in Figure 1. The baseline mean arterial pressure was 87.9 ± 10.2 and 92.2 ± 10.8 in the normal control and hypertensive control groups respectively. There was a significant rise in BP when the drugs (adrenaline, nor adrenaline, dopamine) were administered at doses of 1 µg and 2 µg in the normal control and hypertensive control groups (P < 0.05). The baseline pressure in the prazosin group is 79 ± 5.8, which is low when compared with the control groups. There was also no significant rise in BP when the drugs (adrenaline, nor adrenaline and dopamine) were administered intravenously. The other test groups (both cleistanthin A and B at various doses) also showed an effect similar to prazosin.
In the cleistanthin B 12.5 group, the baseline BP was significantly reduced when compared with other cleistanthin groups (P < 0.05). There was no difference between prazosin and cleistanthin groups except cleistanthin B 12.5 mg/kg indicating that there was no dose‑dependent effect in the groups except cleistanthin B 12.5 mg/kg.
Electrocardiogram changes (data not shown): There was no significant difference among the groups in the ECG parameters before and after the administration of adrenaline, noradrenaline and dopamine.
Discussion
The present study aimed at finding out the antihypertensive effect of cleistanthins A and B showed that the compounds had a significant antihypertensive effect similar to prazosin [Tables 1 and 2].
It was reported that the patients who consumed C. collinus developed uncontrolled hypotension.[3] The hypotensive activity of the extract and the compounds was demonstrated in many animal studies. The individual phytoconstituents (cleistanthins A and B) also showed a dose‑dependent hypotensive effect after direct i.v. administration.[22] The present study differed from the earlier ones in that it demonstrated “antihypertensive” rather than “hypotensive” activity by virtue of the model selected for the present study. However, it failed to produce a dose‑dependent effect unlike the earlier study where the i.v. administration of the compounds elicited a dose‑dependent response. Though it is not possible to give a clear cut reason for the difference, selection of lower doses could have demonstrated the effect since cumulation of the compounds might have occurred in the body due to daily administration for 7 days.
There was no difference among cleistanthin groups with the sole exception of cleistanthin B 12.5 mg/kg group. It indicates that cleistanthins A and B do not exert a dose‑dependent effect. However, cleistanthin B 12.5 mg/kg lowered the mean arterial pressure much more than that by the higher doses (25 and 50 mg/kg) [Figure 1]. No explanation can be offered at this stage as to why higher doses produced lesser effect.
The mechanism of the antihypertensive activity was also evaluated, and both the compounds showed a significant alpha‑adrenergic receptor blocking property similar to prazosin [Figure 1]. Kumar et al. demonstrated the alpha receptor blocking property of the crude extract of C. collinus leaves on the isolated tissues.[23] The direct i.v. administration of the aqueous extract of C. collinus in rats resulted in hypotension, but there was no alpha‑adrenergic receptor blockade. The phytochemical variations of the extract was suggested for the absence of alpha‑adrenergic receptor blocking property.[28]
The molecular docking studies showed that cleistanthins A and B had a significant alpha‑adrenergic receptor interaction similar to prazosin.[29] It was reported that cleistanthins A and B blocked alpha‑adrenergic receptor in guinea pig vas deferens.[24] The present study also picked up similar effect as evident from the failure to record a rise in the mean arterial pressure after the administration of the catecholamines in the cleistanthin treated rats indicating the alpha‑adrenergic receptor blocking property of the latter [Figure 1].
In the present study, the compounds were administered orally for 1‑week, and a reduction in BP could be due to another factor apart from the alpha receptor blocking property. The compounds were reported to exert diuretic effect, and this could also have contributed to the reduction in BP.[14] Since we have not recorded the urine output in this study, the above explanation could remain as speculation only.
There was a significant rise in the end‑diastolic pressure in the control groups after the administration of drugs whereas the standard and the test groups did not show any rise [Figure 2]. The end diastolic pressure, a measure of the left ventricular dysfunction in rats is assessed by the carotid cannulation and inserting the catheter progressively until it reaches the left ventricle. The gold standard for measuring end‑diastolic pressure is through invasive method, but other noninvasive methods like echocardiography can also be used to measure the pressure.[30] However, in the present study such procedures were not carried out, and the values calculated were only predicted values by the software system. Prazosin as an alpha blocker has a tendency to reduce the end‑diastolic pressure.[31] The present study demonstrates the reduction in end‑diastolic pressure by the compounds, which also exert alpha‑adrenergic receptor blockade. Digoxin, a cardiac glycoside, also reduces the end‑diastolic pressure.[32] Though cleistanthins A and B are also glycosides, they do not share the properties of digoxin. The reduction in the end diastolic pressure can be attributed to their alpha‑adrenergic blockade only.
There were no significant changes in the weekly heart rate by noninvasive method [Table 3]. Jose et al. reported that cleistanthus at low doses causes tachycardia and at high doses causes arrhythmias, and cardiac arrest.[33] In the present study, the individual phytoconstituents didn’t show any effect on heart rate and this could be because the crude plant product contains a lot of compounds other than cleistanthins A and B and they might be responsible for the rise in the heart rate.
There were no significant ECG changes indicating that the compounds didn’t have much effect on the electrical activity of the heart. Various case reports on the C. collinus poisoning showed ECG changes like sinus tachycardia, sinus bradycardia, ST depression, T wave inversion, prolongation of QT interval in patients.[34‑36] The direct i.v. administration of the compound cleistanthin A showed a significant increase in RR interval, decrease in heart rate, increase in R amplitude, S amplitude, and ST height and decrease in T amplitude at a dose of 128 µg. Cleistanthin B on direct i.v. administration increased ST height, RR interval and decreased the heart rate.[22] The fact that no changes in ECG were observed in the present study could be attributed to the difference in bioavailability between i.v. and oral routes. It was reported by Maneksh et al. that no evidence of cardiotoxicty was found in rats when the crude extract was given, and the present study also show similar results.[37]
All the previous animal studies which demonstrated the hypotensive effect of either the crude extract or the individual constituents were performed on normal rats. One of the strengths of the present study is it demonstrates the antihypertensive effect of the compounds in DOCA salt model, which is used in the evaluation of antihypertensive agents. Estimation of the levels of cleistanthins in blood could have given a better idea of their bioavailability and pharmacokinetic parameters, which would have enabled us to correlate them with the pharmacodynamic effects. Complex assay procedure and lack of time precluded us from including estimation of the compounds in the present study. Failure to demonstrate the dose‑dependent is a limitation of the study.
Conclusion
Cleistanthins A and B significantly reduce the BP in hypertensive rat models, and the effect is comparable to prazosin. The reduction in BP is due to alpha-adrenergic receptor blocking property of cleistanthins A and B similar to prazosin.
Acknowledgements
The study was supported by the intramural fund from JIPMER institute, Puducherry.
References
- Eswarappa S, Chakraborty AR, Palatty BU, Vasnaik M. Cleistanthus collinus poisoning: Case reports and review of the literature. J Toxicol Clin Toxicol 2003;41:369-72.
- Pinho PM, Kijjoa A. Chemical constituents of the plants of the genus Cleistanthus and their biological activity. Phytochem Rev 2007;6:175-82.
- Subrahmanyam DK, Mooney T, Raveendran R, Zachariah B. A clinical and laboratory profile of Cleistanthus collinus poisoning. J Assoc Physicians India 2003;51:1052-4.
- Thomas K, Dayal AK, Gijsbers A, Seshadri MS, Cherian AM. Oduvanthalai leaf poisoning. J Assoc Physicians India 1987;35:769-71.
- Nampoothiri K, Chrispal A, Begum A, Jasmine S, Gopinath KG, Zachariah A. A clinical study of renal tubular dysfunction in Cleistanthus collinus (Oduvanthalai) poisoning. Clin Toxicol (Phila) 2010;48:193-7.
- Sarathchandra G, Balakrishnamurthy P. Pertubations in glutathione and adenosine triphosphatase in acute oral toxicosis of Cleistanthus collinus: An indigenous toxic plant. Indian J Pharmacol 1997;29:82-5.
- Govindachari TR, Sathe SS, Viswanathan N, Pai BR, Srinivasan M. Chemical constituents of Cleistanthus collinus (Roxb). Tetrahedron 1969;25:2815-21.
- Anjaneyulu A, Ramaiah P, Row L, Venkateshwarlu R, Pelter A, Ward R.New lignans from the heartwood of Cleistanthus collinus. Tetrahedron 1981;37:3641-52.
- Annapoorani KS, Damodaran C, Chandra Sekharan P. Solid-state fluorodensitometric quantitation of arylnaphthalene lignan lactones of Cleistanthus collinus. J Chromatogr 1984;303:296-305.
- Ramesh C, Ravindranath N, Ram TS, Das B. Arylnaphthalide lignans from Cleistanthus collinus. Chem Pharm Bull 2003;51:1299-300.
- Raveendran R, Parasuraman S, Gopal V. Isolation and purification of cleistanthin A and B from the leaves of Cleistanthus collinus Rob. (Euphorbiaceae). Arch Pharm Sci Res 2009; 1:199-202.
- Raveendran R, Parasuraman S, Madhavrao C. GC-MS analysis of leaf extracts of Cleistanthus collinus roxb. (Euphorbiaceae). Int J Pharm Sci 2009; 1:284-6.
- Pratheepa M. GC-MS and in-silico analysis of Cleistanthus collinus for its activity against cancer. Drug Discov 2012;1:23-6.
- Parasuraman S, Raveendran R. Diuretic effects of cleistanthin A and cleistanthin B from the leaves of Cleistanthus collinus in wistar rats. J Young Pharm 2012;4:73-7.
- Prabhakaran C, Kumar P, Panneerselvam N, Rajesh S, Shanmugam G. Cytotoxic and genotoxic effects of cleistanthin B in normal and tumour cells. Mutagenesis 1996;11:553-7.
- Pradheepkumar CP, Shanmugam G. Anticancer potential of cleistanthin A isolated from the tropical plant Cleistanthus collinus. Oncol Res 1999;11:225-32.
- Damodaram P, Manohar IC, Prabath Kumar D, Mohan A, Vengamma B, Rao MH. Myasthenic crisis-like syndrome due to Cleistanthus collinus poisoning. Indian J Med Sci 2008;62:62-4.
- Pradheepkumar CP, Panneerselvam N, Shanmugam G. Cleistanthin A causes DNA strand breaks and induces apoptosis in cultured cells. Mutat Res 2000;464:185-93.
- Nandakumar NV, Pagala MK, Venkatachari SA, Namba T, Grob D.Effect of Cleistanthus collinus leaf extract on neuromuscular function of the isolated mouse phrenic nerve-diaphragm. Toxicon 1989;27:1219-28.
- Tuchinda P, Kumkao A, Pohmakotr M, Sophasan S, Santisuk T, Reutrakul V. Cytotoxic arylnaphthalide lignan glycosides from the aerial parts of Phyllanthus taxodiifolius. Planta Med 2006;72:60-2.
- Parasuraman S, Raveendran R. Computer-aided prediction of biological activity spectra, pharmacological and toxicological properties of cleistanthin A and B. Int J Res Pharm Sci 2010;1:333-7.
- Parasuraman S, Raveendran R, Selvaraj RJ. Effects of cleistanthins A and B on blood pressure and electrocardiogram in Wistar rats. Z Naturforsch C 2011;66:581-7.
- Kumar MR, Ramaswamy S, Jayanthi M, Raveendran R. Alpha-adrenergic receptor blocking effect of Cleistanthus collinus (Roxb.) Benth. And Hook f. leaf extract on guinea pig isolated smooth muscle preparations. Indian J Exp Biol 2011;49:339-42.
- Parasuraman S, Raveendran R. Effect of cleistanthin A and B on adrenergic and cholinergic receptors. Pharmacogn Mag 2011;7:243-7.
- Bockman CS, Jeffries WB, Pettinger WA, Abel PW. Enhanced release of endothelium-derived relaxing factor in mineralocorticoid hypertension. Hypertension 1992;20:304-13.
- Field KJ, White WJ, Lang CM. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim 1993;27:258-69.
- Parasuraman S, Raveendran R. Measurement of invasive blood pressure in rats. J Pharmacol Pharmacother 2012;3:172-7.
- Parasuraman S, Raveendran R. The effects of aqueous extract of Cleistanthus collinus (Roxb.) (Euphorbiaceae) leaves on rat blood pressure. Pharmacognosy Res 2012;4:178-80.
- Parasuraman S, Raveendran R, Vijayakumar B, Velmurugan D,Balamurugan S. Molecular docking and ex vivo pharmacological evaluation of constituents of the leaves of Cleistanthus collinus (Roxb.) (Euphorbiaceae). Indian J Pharmacol 2012;44:197-203.
- Saraiva RM, Kanashiro RM, Antonio EL, Campos O, Mois�s VA. Rats with high left ventricular end-diastolic pressure can be identified by Doppler echocardiography one week after myocardial infarction. Braz J Med Biol Res 2007;40:1557-65.
- Prasad K, O?Neil CL, Bharadwaj B. Effect of prolonged prazosin treatment on hemodynamic and biochemical changes in the dog heart due to chronic pressure overload. Jpn Heart J 1984;25:461-76.
- Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation 2004;109:2942-6.
- Jose VM, Anand KN, Jeyaseelan L, Ernest K, Kuruvilla A. Effect of potassium channel modulators on toxicity of Cleistanthus collinus. Indian J Exp Biol 2004;42:81-5.
- Chrispal A. Cleistanthus collinus poisoning. J Emerg Trauma Shock 2012;5:160-6.
- Benjamin SP, Fernando ME, Jayanth JJ, Preetha B. Cleistanthus collinus poisoning. J Assoc Physicians India 2006;54:742-4.
- Shankar V, Jose VM, Bangdiwala SI, Thomas K. Epidemiology of Cleistanthus collinus (oduvan) poisoning: Clinical features and risk factors for mortality. Int J Inj Contr Saf Promot 2009;16:223-30.
- Maneksh D, Sidharthan A, Kettimuthu K, Kanthakumar P,Lourthuraj AA, Ramachandran A, et al. Cleistanthus collinus induces type I distal renal tubular acidosis and type II respiratory failure in rats. Indian J Pharmacol 2010;42:178-84.