Effects of Vacuum Liquid Chromatography (Chloroform) Fraction of the Stem Bark of Alstonia boonei on Mitochondrial Membrane Permeability Transition Pore
Citation: Olanlokun OJ, Oyebode TO, Olorunsogo OO. Effects of Vacuum Liquid Chromatography (Chloroform) Fraction of the Stem Bark of Alstonia boonei on Mitochondrial Membrane Permeability Transition Pore. J Basic Clin Pharma 2017;8:221-225.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Background: The Mitochondrial Membrane Permeability Transition (MMPT) pore is an important target for the development of cytotoxic drugs because the release of cytochrome c and the irreversible opening of the pore is a point of no return for apoptosis to take place. A. boonei is a perennial plant that is quite ubiquitous in rain forest and sub-Saharan regions and it is used in folklore medicine as an anti-malarial decoction. Objective: To assess the effects of Vacuum Liquid Chromatography (VLC) chloroform (100%) fraction purified from the stem bark extract of A. boonei on MMPT pore of mitochondria isolated from livers of male rats in the presence and absence of added calcium ions. Materials and Methods: The in vitro effects of the VLC chloroform fraction on MMPT were assessed by monitoring the amplitude swelling spectrophotometrically as decreases in absorbance at 540 nm. Results: The MMPT pore was opened by six folds in the presence of calcium than in the absence of calcium. Spermine, a standard inhibitor of the pore almost completely reversed the effect of calcium thus indicating that the mitochondria were intact ab initio and suitable for use. Although calcium-induced pore opening was significantly inhibited by the VLC fraction in a concentration-dependent manner by 78.24, 88.55, 90.27 and 91.04% at 2.5, 5, 10 and 20 μg/ml, respectively, pre-incubation of mitochondria for 5, 10 and 15 minutes on ice and in the absence of calcium caused MMPT pore opening by two, five, and four folds, respectively, at 1.25 μg/ml and two, five and two folds, respectively at 2.5 μg/ml. Pre-incubation for these periods at 5 μg/ml caused the pore to open by two, seven and five folds, respectively, and four, eight and four folds at 10 μg/ml, respectively. Conclusion: These findings suggest that the bioactive agents in this fraction may find use in situations that require modulation of apoptosis.
Keywords
Alstonia boonei, apoptosis, calcium, chromatography, mitochondria, pre-incubation
Introduction
Mitochondria play an important role in cell death because of its prominent feature in apoptotic signal transduction and amplification.[1,2] The complex role of mitochondria in apoptosis came into focus when biochemical studies identified several mitochondrial proteins that are able to activate cell death directly via mitochondrial-mediated pathway. [3] Prior to apoptosis, mitochondria suffer specific damages that result in release of cytochrome c, the sole water-soluble component of the electron transfer chain, and this can potentially halt the electron transfer leading to failure in maintaining the mitochondrial membrane potential that is needed in ATP synthesis.[4] It has been established that once released, cytochrome c binds to apoptotic protease activator-1 (APAF-1) in the presence of ATP or dATP and forms a complex that activates pro-caspase-3 and -7.[5]
In cell-free systems, it has been demonstrated that mitochondria are necessary components of the cytosolic fraction to produce apoptotic features.[6] Loss of mitochondrial ATP synthesis has been reported in cells after growth-factor deprivation.[7] In this apoptotic cells, cellular ATP content decreased when cytosolic ADP and intermembrane creatinine phosphate concentration increase. The MMPT is a phenomenon whereby the exquisitely controlled permeability of the inner membrane is disrupted and mitochondria become nonselectively permeable to solutes up to 1.5 kD.[8,9] Associated with this is the rapid equilibration of solutes across the mitochondrial membranes leading to depolarization of membrane potential, osmotic swelling, and release of potential apoptogenic actors including cytochrome c.[10] Thus, the MMPT-induced loss of mitochondrial function, release of cytochrome c, and the resultant ROS generation account for cell death.
The MMPT pore is thought to be a multi-protein complex which assembles at the sites of contact between the inner and outer mitochondrial membranes.[11] The pore normally remains closed, but can open under conditions of cellular stress with dire consequences. When the MMPT pore opens, the permeability barrier of the inner membrane becomes disrupted, all small molecular weight solutes move freely across the membrane and as a result, they exert a colloidal osmotic pressure that causes mitochondria to swell and the unfolding of the cristae allows the matrix to expand without rupture of the inner membrane, the outer membrane will break and lead to the release of proteins in the inter-membrane space such as cytochrome c. Also, the inner membrane becomes freely permeable to protons. This uncouples oxidative phosphorylation, causing the proton-translocating ATPase to reverse direction and so actively hydrolyse ATP, rather than synthesize it.[12]
Under such conditions, intracellular ATP concentrations rapidly decline, leading to the disruption of ionic and metabolic homeostasis and the activation of degradative enzymes such as phospholipases, nucleases, and proteases. The key factors responsible for MMPT pore opening are: mitochondrial calcium overload (i.e., when mitochondrial matrix [Ca2+] is greatly increased), presence of pro-oxidants, chemotherapeutic agents and proapoptotic Bcl-2 family members (e.g., Bax, Bak, and Bid).[13] The pivotal role played by mitochondria in cell death has made them a vulnerable target for experimental and pharmacological intervention.[14] It has been suggested that certain bioactive agents in medicinal plants could elicit chemo-protective and therapeutic effects through the induction or inhibition of the opening of the MMPT.[15]
A. boonei (De Wild) is a very large deciduous, tropical forest tree that belongs to the dogbane family, Apocynaceae which consists of about 50 species widely distributed in the continents of Africa, Asia and America.
It is native to tropical West Africa, with a range extending into Ethiopia and Tanzania. Its common name in the English timber trade is cheese wood, pattern wood or stool wood while its common name in the French timber trade is “emien” (derived from the vernacular of the Ivory Coast). Its name corresponds to the latest revision in the plant list. Parts of the plant are employed for the treatment of a variety of ailments in Africa and the stem bark has been listed in the African Pharmacopoeia as an anti-malarial drug.[16-19] The stem bark of A. boonei is used in traditional medicine to treat fever, painful micturition, insomnia, chronic diarrhea, rheumatic pains, as anti-venom for snake bites, in the treatment of arrow poisoning and malaria.[20-24] An infusion of the stem bark in hot water is used in the treatment of neonatal haemolytic jaundice in Nigeria.[25] The ethanol extract of the leaves have also been found to have intrinsic antimalarial properties and safe for use.[26] Also, the anti-inflammatory and anti-arthritic properties of the root bark of A. boonei had been demonstrated.[27,28] The phytomedical use of this plant may not be unconnected with various phytochemicals, such as alkaloids, flavonoids, saponnins and polyphenols that are found in various solvent extracts and fractions of A. boonei.
Many studies have described the proapoptotic properties of dietary polyphenols in a variety of human cancer cell lines derived from colon, prostate, lung, breast cancers and leukemia.[25] However, there is paucity of information about the modulation of the intrinsic pathway of apoptosis by the phytochemical constituents of the stem bark of A. boonei. This research therefore was carried out to determine the modulatory effects of the VLC chloroform fraction of A. boonei stem bark on mitochondrial membrane permeability transition pore both in the presence and absence of calcium and also to determine the effect of pre-incubation of mitochondria in the VLC chloroform fraction on mitochondrial membrane permeability transition.
Materials and Methods
The maceration method of extraction of medicinal plant was used. Briefly, the stem bark of A. boonei (De wild) was peeled from bushes in various surroundings of the Ekiti State University, Ado-Ekiti. Samples were authenticated and identified at the Herbarium, Plant Science Department, Ekiti State University and a specimen (Voucher No. UHAE 013) was deposited in the Herbarium. The stem bark peels were air-dried at room temperature to avoid possible degradation or denaturizing of their active compounds. The air-dried stem bark of A. boonei was blended to powder using an electric blender. This was stored in a glass container. Thereafter, 5.73 kg of blended air-dried stem bark was soaked in 10 litres of methanol for 72 hours at room temperature. It was continually stirred after each 24 hours. After 72 hours, the mixture was filtered and the filtrate was concentrated using rotary evaporator. The crude methanol extract concentrate weighed 53 g.
About 250 ml of distilled water was added to 20 gm of the dried methanol extract to form slurry; which was partitioned repeatedly with n-hexane in a separating funnel flask until exhaustion. The marc remaining was partitioned again with chloroform until exhaustion. The remaining marc was finally partitioned with ethyl acetate until exhaustion and the aqueous fraction was collected. All these fractions were concentrated under pressure using rotary evaporator at 40°C for n-hexane, chloroform, ethyl acetate and aqueous residue and subsequently freeze dried to obtain the n-hexane fraction (HF), chloroform fraction (CF), ethyl acetate fraction (EF) and the aqueous fraction (AF). The partitioned methanol extract yielded 14.4%, 8.2%, 3.1% 08 and 12.6% for the HF, CF, EF and AF respectively.
Vacuum Liquid Chromatography Purification Procedure
The chloroform fraction was further purified using the Vacuum Liquid Chromatography (VLC). The pre-washed sintered glass Buchner was further cleaned with concentrated H2SO4 to remove impurities from the sieve. The column was then packed three-quarter full with silica gel 60 (0.040-0.063 mm, MERCK). The column was then placed on a conical flask Buchner and connected to the vacuum pump. The pump was switched on and n-hexane solvent was topically applied to the column. This was done to further pack the column. Thereafter, 12 gm of the chloroform fraction was adsorbed on 8 g of silica gel 60 (0.040-0.063 mm, MERCK). The gel-sample mixture was stirred until a homogenous mixture was obtained. The mixture was air-dried and applied to the top of the column and with the pump switched on, the first solvent system, 100% n-hexane was added to the column. The flow rate was 25 ml/min. This was done with 750 ml of the n-hexane solvent. Next solvent system, n-hexane: chloroform (1:1) was made by mixing 50 ml of n-hexane with 50 ml of chloroform and the column was eluted with the solvent system. This was done until there was a complete elution of the fraction from the column. The column was further washed with chloroform only (100%) and lastly with chloroform: methanol (1:1). The fractions obtained were concentrated at 40°C using rotary evaporator and transferred into pre-weighed all-glass sample bottles with glass stopper and labelled. Thin Layer Chromatography (TLC) fractions were carried out in order to assess the purity and also to identify the phyto chemicals present in each of the fractions gotten from the solvent systems.
Isolation of Rat Liver Mitochondria
Low ionic strength mitochondria were essentially isolated from normal healthy male wistar albino rats using the method of Johnson and Lardy. [26] Briefly the animals were sacrificed by cervical dislocation and the liver was quickly excised, trimmed to remove excess tissue. This was homogenized in buffer C (210 mM Mannitol, 70 mM Sucrose, 5 mM Hepes-KOH (pH 7.4) 1 mM EGTA). The homogenate was centrifuged in a refrigerated centrifuge at 2,500 rpm twice at five minutes each to remove cell debris and hydrolytic enzymes.
Mitochondria were pelleted at 13,000 rpm for ten minutes. These were washed twice for ten minutes each at 12,000 rpm with buffer D (210 mM mannitol, 70 mM sucrose, 5 mM Hepes-KOH (pH 7.4), 0.50% bovine serum albumin). Pelleted mitochondria were suspended in Mannitol Succinate Hepes (MSH) buffer (210 mM mannitol, 70 mM sucrose and 5 mM Hepes-KOH (pH 7.4).
Mitochondrial Swelling Assay
Mitochondrial swelling was assayed according to the method of Lapidus and Sokolove.[27] Briefly, were pre-incubated in MSH buffer in the presence of 0.8 μM rotenone for 3 minutes in a 2.50 quartz glass cuvette at 30°C. 0.3 μM calcium was added immediately after the 3 minutes incubation period and 5 mM sodium succinate was added 30 seconds after to energise the medium. Absorbance values were read as a light scattering effect over a period of 12 minutes at 30 seconds interval at 540 nm in a CamSpec M106 spectrophotometer. The spectrophotometer was blanked using 2.50 ml of the MSH buffer. Different concentrations of the extracts (VLC fractions) were used. In this experiment, 10, 20, 40 and 80 μl of the extracts were added in place of distilled water added in control. The concentrations of the extracts used were 2.5 μg, 5 μg, 10 μg and 20 μg.
Pre-Incubation of Mitochondria in the VLC Fraction
Mitochondria were pre-incubated with varied VLC chloroform fraction concentrations (1.25, 2.50, 5.0 and 10 μg/ml) for 5, 10 and 15 minutes for each concentration. A corresponding amount of preincubated mitochondria plus the VLC chloroform fraction was added to the assay medium of the MSH buffer and 8 μM rotenone. The whole assay medium was then incubated for 3½ minutes in a glass cuvette at 30°C. Later, 25051 mM sodium succinate was added 30 seconds after to energize the mitochondrial swelling. The light scattering effect was measured at 540 nm over a period of 12 minutes at 30 seconds interval in a Cam Spec M106 spectrophotometer.
Results
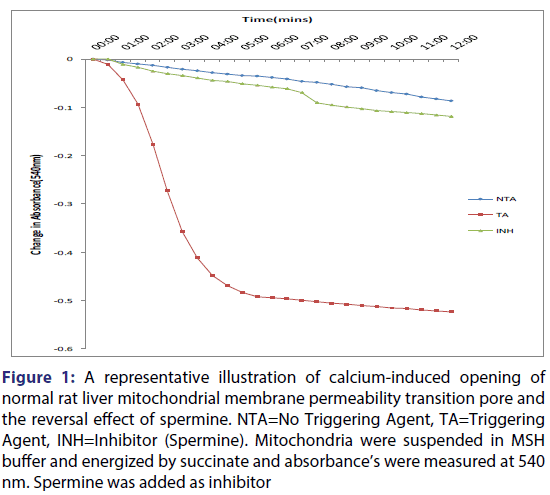
The results for the assessment of Ca2+ induced mitochondrial membrane permeability transition pore opening and inhibition by spermine is presented in Figure 1. The data obtained showed that mitochondrial membrane was intact in the absence of Ca2+ as there was no appreciable decrease in absorbance over a period of 12 minutes, whereas in the presence of Ca2+, the mitochondrial membrane permeability transition pore was significantly opened by six folds. This was shown by the large amplitude swelling which indicated the susceptibility of the mitochondria to pore opening in the presence of Ca2+.
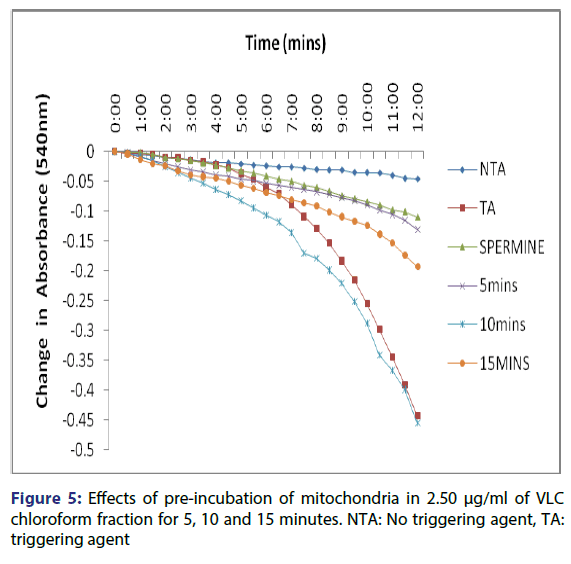
Figure 1: A representative illustration of calcium-induced opening of normal rat liver mitochondrial membrane permeability transition pore and the reversal effect of spermine. NTA=No Triggering Agent, TA=Triggering Agent, INH=Inhibitor (Spermine). Mitochondria were suspended in MSH buffer and energized by succinate and absorbance’s were measured at 540 nm. Spermine was added as inhibitor
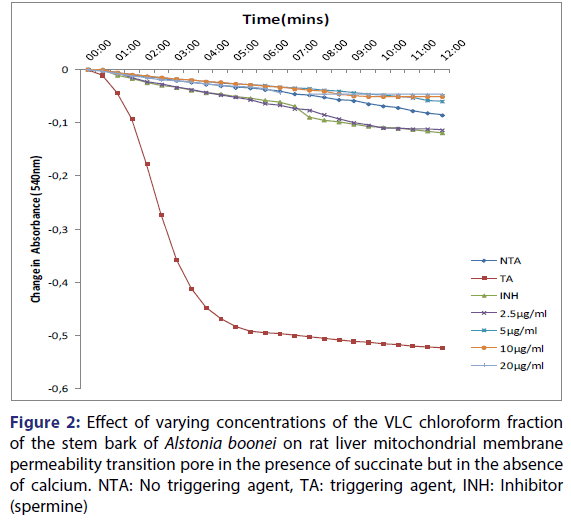
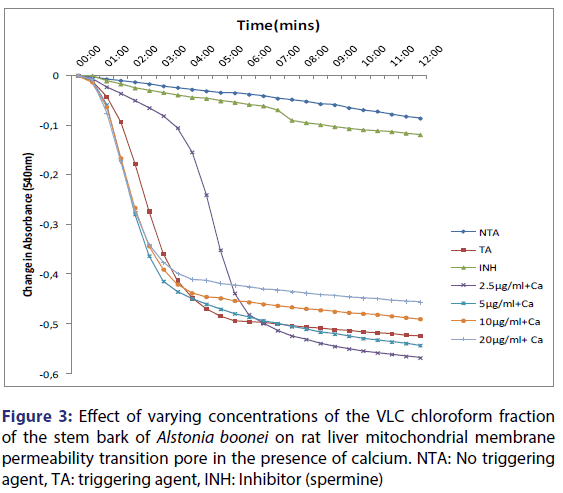
Furthermore, spermine inhibited calcium-induced mitochondrial swelling in normal rat liver mitochondria. Calcium-induced pore opening was significantly inhibited by the VLC fraction in a concentration-dependent manner, by 78.2, 88.6, 90.3 and 91.0% at 2.5, 5, 10 and 20 μg/ml, respectively [Figure 2]. The assessment of the MMPT in the presence of other VLC fractions: n-hexane: chloroform (1:1), and chloroform: methanol (1:1) did not have any significant effect. Figure 3 showed the effects of the various concentrations of the extracts in the presence of calcium. It could be seen that at lower concentrations (1.25 and 2.5 μg/ml) chloroform fractions further opened the MMPT pore more than at higher concentrations (5 and 10 μg/ml).
Figure 2: Effect of varying concentrations of the VLC chloroform fraction of the stem bark of Alstonia boonei on rat liver mitochondrial membrane permeability transition pore in the presence of succinate but in the absence of calcium. NTA: No triggering agent, TA: triggering agent, INH: Inhibitor (spermine)
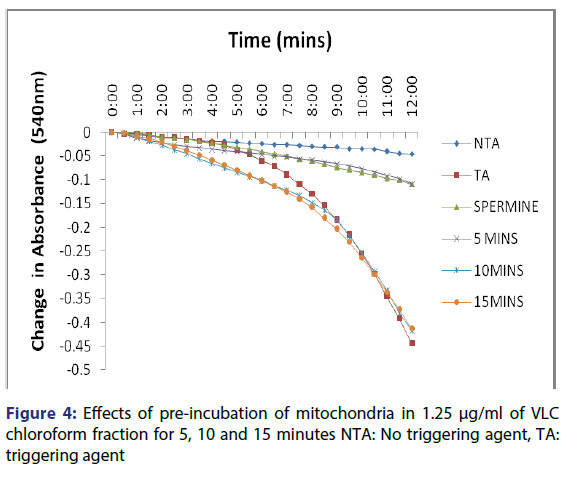
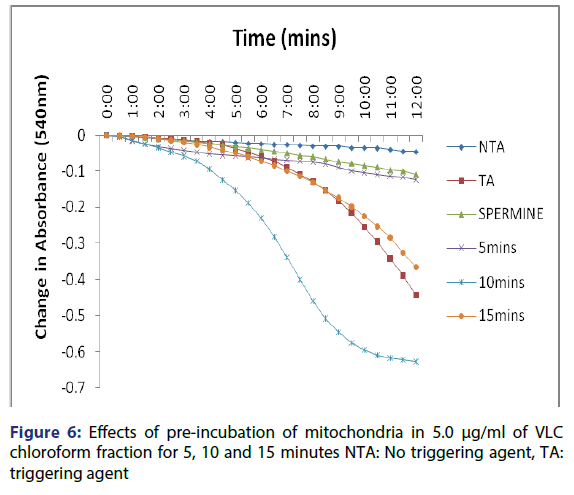
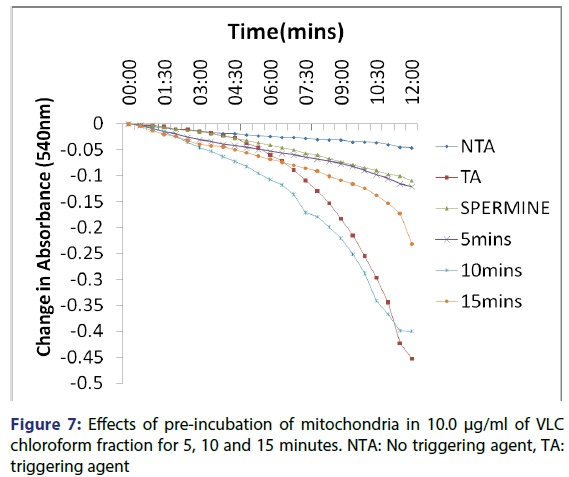
This showed that calcium is needed for the lower concentrations to open the pore and that this fraction in the presence of calcium opened the MMPT pore more than when only calcium was used. Figure 4 showed the effect of pre-incubation of the mitochondria in 1.25 μg/ ml of the VLC chloroform fraction for 5, 10 and 15 minutes. From the D, permeabilization of the mitochondrial membrane increased at 10 minute better than at 5 minute and a reversal was noticed at 15 minutes showing that the VLC fraction may be interacting with the mitochondria in a non-specific manner. The same trend was noticed at 2.5, 5 and 10 μg/ml [Figures 5-7] but at a higher swelling rate (1.25< 2.5< 5< 10 μg/ml).
Pre-incubation increased the interaction between the VLC chloroform fraction of the stem bark of A. boonei and mitochondria. The pore opening was also found to be concentration dependent.
Discussion
Apoptosis is a potent defence mechanism by which mutated cells can be destroyed, thus preventing cancer occurrences.[28] Plants have been good sources of secondary metabolites such as flavonoids that have antioxidant properties and have been used for the treatment and prevention of diseases that are related to oxidative stress while alkaloids and terpenes-derived drugs such as vincristin and artemisinin have also been used in the treatment of cancer and malaria respectively. These secondary metabolites are soluble in different polar and non-polar solvents. Therefore, methanol was used for the crude extraction because of its amphipathic nature and n-hexane, chloroform, ethyl acetate and water were used for the partitioning because of the varying solubility of the various phytochemical constituents from the methanol extract in them and the partitioning was done in order of increasing polarity. The mitochondrial membrane permeability transition (MMPT) pore is an important pharmacological target for the development of cytotoxic drugs because the irreversible opening of the pore which is followed by the concomitant release of cytochrome c is a point of no return for apoptosis to take place.
In many diseases, aberrant regulation of apoptosis is the central abnormality. For example, resistance of cells to apoptosis is thought to be responsible for many types of cancer, and identification of the molecular alterations responsible for such cell immortalization is an important area in cancer research.[29] Although details of the definition of apoptosis vary among investigators, there is general agreement that apoptosis is a cell death process involving caspase activation and a lack of cell swelling with maintenance of organelle (mitochondria and endoplasmic reticulum) integrity.
In this study, the effects of the Vacuum Liquid Chromatographic (VLC) chloroform (100%) fraction purified from the stem bark extract of A. boonei on MMPT pore of mitochondria isolated from livers of male rats in the presence and absence of exogenous calcium were assessed by monitoring amplitude swelling spectrophotometrically as decreases in absorbance at 540 nm. This particular fraction was used for this study because in our previous study, it has the highest antiplasmodial activity against P. berghei-induced malaria in mice showing that it contains certain phytochemical(s) of medicinal value.[24]
The qualitative screening of this fraction showed that it contained phytochemicals such as alkaloids, saponins, tannins, steroids, terpenoids, cardiac glycosides and flavonoids. Figure 1 showed the traces for intact mitochondria in the absence of calcium, the large amplitude swelling induced by calcium and its reversal by spermine. Spermine, a known inhibitor of mitochondrial membrane permeability transition, was used as a standard and was confirmed to inhibit mitochondrial swelling significantly which agrees with the reports of Lapidus and Sokolove.[30] Increased matrix Ca2+ in combination with a triggering agent induces a permeability transition of the inner mitochondrial membrane.[31-35] The ability of exogenous calcium to induce the pore opening and spermine to reverse the pore opening showed that the mitochondria used for this work were intact ab initio and are suitable for use. Figure 2 showed that calcium-induced pore opening was significantly inhibited by the VLC fraction in a concentration-dependent manner by 78, 89, 90 and 91% at 2.5, 5, 10 and 20 μg/ml, respectively. These results suggest that the VLC chloroform (100%) fraction in purified form may contain active principles that may find therapeutic use in situations that require the reduction or inhibition of excessive cell death such as neurodegenerative diseases. Figure 3 showed the reversal effect of the varying concentrations of the VLC chloroform fraction on calciuminduced pore opening. Although, calcium is a standard inducer of the opening of the pore, the chloroform fraction reversed this effect in concentration dependent manner, the highest inhibition noticed at the highest concentration.
The preliminary phyto chemical analysis of the stem bark of A. boonei showed presence of alkaloid, phenolic, flavonoids, tannins, steroids, glycosides and saponins. However, all these chemicals were not extractable in one solvent. The availability of specific phyto chemicals in plant gives it specific medicinal properties. Therefore, the biochemical activity of the chloroform fraction on the mitochondrial membrane permeability transition pore may be due to the class of phyto chemicals extractable by the solvent. Phyto chemicals such as alkaloids, phenolics, flavonoids and glycosides are extractable with chloroform,[31] it may be that the modulatory effects of the VLC chloroform fraction on MMPT pore may be due to any of these phyto chemicals. Also, synergistic effects of the phyto chemicals may not be ruled out.
The ability of these polar compounds to interact with the membrane polar head groups may further increase the concentrations at the water-lipid interface causing a higher stability of the membrane and thus preventing Ca2+-induced pore opening. On the other hand, if these compounds are able to penetrate the inner membrane, then the inner membrane becomes permeabilized leading to uncoupling of the oxidative phosphorylation mechanism and aggregation of the presumed components of the MMPT pore such as ANT, VDAC and Cyp-D.
The assessment of the effects of other VLC fractions such as n-hexane: chloroform (1:1), and chloroform: methanol (1:1) on the MPT pore opening did not have any significant effect both in the presence or absence of exogenous calcium. This could be that the phyto chemicals present in these fractions could not present any presumable effect on the mitochondrial membranes. Our overall findings suggest that the inductive effect of the purified fraction on pre-incubation with mitochondria is by far greater than its inhibitory effect in vitro. Upon pre-incubation of mitochondria in smaller concentrations of the VLC fraction for 5, 10, and 15 minutes in the absence of calcium, MMPT pore opening was largely induced by the VLC fractions. The pore opened by two, five and four folds respectively at 1.25 μg/ml, [Figure 4] and two, five and two folds [Figure 5] respectively at 2.5 μg/ml. preincubation for these periods at 5 μg/ml caused the pore to open by two, seven and five folds, respectively [Figure 6] and by four, eight and four folds at 10 μg/ml [Figure 7] respectively.
Although, it may be surprising that the same stem bark extract and more so the same partially purified VLC fraction that inhibited calciuminduced pore opening could also be inducing the pore opening in the absence of calcium-triggering agent albeit under different conditions, this observation is plausible because further interaction of the extract for longer periods may cause the extract to permeate the outer membrane of the mitochondria, eventually causing a leaky membrane that gives the observed swelling. It is possible also that this effect may not have a direct relationship with the modulation of the mitochondrial permeability transition pore.
We hereby conclude that the VLC fraction from the stem bark of A. boonei modulates the mitochondrial membrane permeability transition pore and that this feature can be explored for use.
Conflict of Interest
No competing financial interests exist.
REFERENCES
- Green DR, Reed JC. Mitochondria and apoptosis. Science 1998;281:1309-12.
- Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardio protection. Cell Physiol. Biochem 2007;20:1-22.
- Li LY, Lu OX, Wang X. Endonulcease G (enndo G) is an apoptotic DNase when released from mitochondria. Nature 2001;412:995-9.
- Wang X. The expanding role of mitochondria in apoptosis. Genes and Development 2001;15:2922-53.
- Kroemer G. Introduction: mitochondrial control apoptosis. Biochimie 2002;84:103-4.
- Newmayer DD, Farschon DM, Reed JC. Cell-free apoptosis in xenopus egg extract: inhibition of Bcl2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353-64.
- Vander-Heiden MG, Thompson CB. Bcl2 protein regulators of apoptosis or of mitochondrial homeostasis? Nat. Cell Biol 1999;1:E209-E16.
- Bernardi P, Vassanelli S, Veronese P. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J. Biol. Chem 1992;267:2934-9.
- Zoratti M, Szabo I, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim. Biophys. Acta 2005;1706:40-52.
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med 2000;6:513-9.
- Bernadi P. Mitochondrial transport of cations: channels, exchangers and permeability transition. Physiol Rev 1999;79:1127-55.
- Zou H, Henzel WJ, Liu X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 1997;90:405-13.
- Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. Journal of Biological Chemistry 1976;251:5069–77.
- McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Commun 1997;239:357-66.
- Tascilar M, de Jong FA, Veinweij J. Complementary and alternative medicine during cancer treatment: beyond innocence. Oncologist 2006;11:732-41.
- Halestrap AP, Kerr PM, Javadov S. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim Biophys Acta 1998;1366:79-94.
- Irvine FR. The Woody Plants of Ghana. Oxford University Press, London, UK. 1961;868.
- Ojewole JAO. Studies on the pharmacology of echitamine, an alkaloid-from the stem bark of Alstonia boonei L. (Apocynaceae). Int J Crude Drug Res 1984;22:121-43.
- Iwu MM. Handbook of African Medicinal Plants. CRC Press, Boca Raton, FL, USA 1993;116-8.
- Faparusi SI, Bassir O. Triterpenes from Alstonia boonei. Phytochemistry 1972;11:3083-4.
- Oliver-Bever B. Medicinal Plants in Tropical West Africa. Cambridge University Press, London, 1986;89-90.
- Kweifo-Okai G, Bird D, Field B. Anti-inflammatory activity of a Ghanaian herbal preparation III. J Ethnopharmacol 1995;46:7-15.
- Asuzu IU, Anaga AO. Pharmacological screening of the aqueous extract of A. boonei stems bark. Fitoterapia 1991;63:411-7.
- Olanlokun JO, Bolaji OM, Agbedahunsi JM. Therapeutic effects of various solvent fractions of Alstonia boonei (apocynaceae) stem bark on Plasmodium berghei-induced malaria. Afr J Med Sci 2012;41:27-33.
- Gbadamosi IT, Obogo SF. Chemical constituents and in vitro antimicrobial activities of five botanicals used traditionally for the treatment of neonatal jaundice in Ibadan, Nigeria. Nature and Science 2013;11:130-5.
- Olayinka FO, Maganda V. In vivo activity of ethanolic extract of Alstonia boonei leaves against Plasmodium berghei in mice. Journal for Worldwide Holistic Sustainable Development 2015;1:60-8.
- Kweifio-Okai G, Carroll AR. Anti-arthritic effect of lupeol acetate. Phytotherapy Research 1993;7:213-15.
- Rajic A, Kweifio-Okai G, Macrides T. Inhibition of serine proteases by anti-inflammatory triterpenoids. Planta Medica 2000;66:206-10.
- Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem 2007;18:427-42.
- Johnson D, Lardy H. Isolation of liver or kidney mitochondria. Methods in Enzymology 1967;10:94-6.
- Lapidus RG, Sokolove PM. Inhibition by spermine of the inner membrane permeability transition of isolated rat heart mitochondria. FEBS Lett.1992;13:314-8.
- Wyllie AH. Apoptosis and carcinogenesis. Eur J Cell Biol 1997;73:189-97.
- Reed J. Mechanism of apoptosis avoidance in cancer. Curr Opin Oncl 1999;11:68-75.
- Lapidus RG, Sokolove PM. Spermine inhibition of the permeability transition of isolated rat liver mitochondria: An investigation mechanism. Arch Biochem Biophys 1993;306:246-53.
- Syed I, Suradkar SS, Koche DK. Phytochemical analysis of Leonotis nepetifolia (L.) R. BR., a wild medicinal plant of lamiaceae. Bioscience Discovery 2012;3:196-7.