Effects of Quercetin and Hesperetin on MCF-7 Cell Proliferation by Using Real-Time Cell Analyzer
- *Corresponding Author:
- Dr. Gülsüm Tekin
Department of Biochemistry, Faculty of Medicine, Selcuk University, Alaeddin Keykubad Campus, 42075 Selcuklu/Konya, Turkey.
E-mail: tekinglsm@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Plant-derived flavonoids have recently interested for the researchers, due to antioxidant and anti-proliferative properties. Cell culture and experimental animal studies have shown to cancer-preventive effects of flavonoids in carcinogenesis. However, some of the biological activities of flavonoids are still unclear. Quercetin and Hesperetin are flavonoid compounds which are evaluated as inhibitors of proliferation of various cancer cells. In our study, we investigated the mechanisms for the effects of Quercetin and Hesperetin on with different concentrations and durations to cell proliferation in MCF-7. Although common methods were used in previous studies the microelectrode based method was used in our proliferation analyze. Materials and Methods: A real-time cell analyzer was used to assess the effects of various doses of Hesperetin (10-175 μM) and Quercetin (125-250 μM) on the proliferation of MCF- 7. Changes in the number of cells were observed continuously every 15 minutes during the experimental period in special cell culture flasks that containing microelectrodes. Results: Hesperetin (175 μM) inhibit the MCF-7 cells proliferation rate of 52.8% compared to the control for 72 h and inhibit cells rate of 60.6% for 96 h after the treatment (P<0.05). Quercetin (250 μM) inhibit MCF-7 cells rate of 70.4% compared to control for 72 h and inhibit cells rate of 81.1% for 96 h after the treatment (P<0.05). Conclusions: As a result, Hesperetin and Quercetin inhibit the human breast cancer cells dose-time dependent manner by real-time cell analyzer. xCELLigence is more comfortable, useful and higher of specificity than the other cell viability analyze methods. As a result, hesperetin and quercetin may be useful therapy or prevention on breast cancer prognosis
Key words
Real-time cell analyzer, hesperetin, MCF-7, proliferation, quercetin
Introduction
Breast cancer is the most common malignancy in women, and is a leading cause of cancer death in women worldwide according to International Agency for Research on Cancer.[1] Several studies report that food products containing flavonoids reduce risk for various cancers including breast cancer. Flavonoids are a group of naturally-occurring, low molecular weight compounds that are widespread in plants or in many foods.[2,3] They are prominent components of citrus fruits and other food sources and are in many countries regularly consumed in a diet. Flavonoids are found in fruits, vegetables, herbs, spices, aromatic plants, tea, red wine[4] as well as stems and leaves of plants.[5]
Flavonoids subdivided into six classes including flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins and isoflavones. Hydroxyl groups which are active in flavonoids, give rise to subdiving of flavonoids and provide characteristic property of them.
These compounds, which have therapeutic effects,[6] may act as natural antitumor agents as chemical inhibitors.[7] Several mechanisms of action have been proposed to explain in vivo and in vitro flavonoid antiproliferative actions such as induction of apoptosis and cell cycle, inhibit cell proliferation, kinase activity and differentiations of cell[3,8-14] and flavonoids may reduce the risk of developing cancer and cardiovascular disease[7,8,15]. Previous studies showed that flavonoids have affected anticancer activities including effective for colon, breast, lung, leukemia, prostate cancers.[1,8,16-21]
Hesperetin (3’,5,7-trihydroxy-4’-methoxyflavone) is a flavonone subgroup of flavonoids. Hesperetin is the aglycone of hesperidin (hesperetin-7-O-rutinosit) in nature and it is found in citrus species[8,18] and is consumed in many countries regularly, especially in Turkey which is Mediterranean country. This polyphenol were observed to inhibit the proliferation human breast cancer cells because of antiestrogenic effect in culture medium[8] Quercetin (3,3’,4’,5,7-pentahidroksiflavon) a member of polyphenolic flavonoids, is one of the most prominent dietary antioxidants and covers a large class of flavonoids.[22] It is found in various vegetables and fruits especially apple and onion, red wine, grapefruit, black tea, raspberries, blueberries, cherries.[5,6] Several epidemiological studies have supported that consumption of a diet rich in containing low-fat and high-fiber of whole grain foods, vegetables and fruits may be associated with a reduced risk of[7,15] various type of cancers.[1,16,17] The major purpose of the present study is to investigate the possible anti-carcinogenic and anti-estrogenic effects of hesperetin and quercetin with various concentrations and durations through the inhibition of cell proliferation in human breast cancer cell line MCF- 7 by a real-time cell analyzer which is a different and more reliable method than the others. Therefore, developing new strategies for therapy of breast cancer progression and overcoming drug resistance represents a major challenge.
Methods and Materials
Cell culture
The breast cancer cell line MCF-7 was obtained by Dr. Abdulkerim Bedir, Medical Faculty of Ondokuz Mayis University, Samsun, Turkey. The cells were placed into tissue culture flasks under a humidified 5% CO2 and 95% air maintained at 37°C atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, v/v), 1% penicillin (100 U/ml)-streptomycin (100 mg/ml) and 1% glutamine (100 mg/ml). The cells were passaged with 0.25% trypsin and 0.1% ethylene diamine tetra acetic acid (EDTA) after reaching 80% of confluency.
Cell proliferation analyzes
Effect of hesperetin and quercetin on MCF-7 cell proliferation was determined by real time cell analyzer (xCELLigence, Roche Diagnostics GmbH, Penzbeerg, Germany) After seeding 200 μl of the cell suspensions in DMEM containing 10% FBS into the wells (10.000 cells/well) of the E-plate 16. Cells were allowed to adhere to the E-plate for 24 h and media were removed from the well. A 200 μM stock solution of hesperetin was prepared in DMSO (0.1%). The cells were treated with different doses of hesperetin (10, 50, 100, 150, 175 μM). Then the hesperetin was added in various concentrations to cells at following every 48 h. The changes of MCF-7 cell proliferation were monitored every 15 min for a period of 144 hours by xCELLigence device. A 250 μM stock solution of quercetin was prepared in DMSO (dimethyl sulfoxide 0.1%). The same pre-treatment was made for different dose groups of quercetin (125, 150, 175, 200, 225, 250 μM). The quercetin was added in various concentrations to cells at following every 48 h. The changes of MCF-7 cell proliferation were monitored every 15 min for a period of 144 hours. Cell proliferation experiments were performed triplicate.
Cell viability analyze by xCELLigence system
The xCELLigence system (xCELLigence, Roche Diagnostics GmbH, Penzbeerg, Germany) was performed to evaluate cell viability. The xCELLigence system comprises 4 components: the impedance based real-time cell analyzer (RTCA), the RTCA a device station, the RTCA computer with integrated software, and three disposable E-plates 16. Electrical impedance are measured across combined with microelectrodes integrated on the bottom of tissue culture E-Plates.[23] The xCELLigence system allows for label-free and dynamic monitoring of cellular phenotypic changes in real time using impedance.[24] Impedance measured between electrodes in an individual well depends on electrode geometry, ionic concentration in the well, and whether there are cells attached to the electrodes.[25,26] The impedance measurement, which is displayed as cell index (CI) value, provides quantitative information about the biological status of the cells, including cell number, viability, and morphology. Therefore, we have preferred the xCELLigence system, because it allows for maximal sensitivity for the detection of the cells with relatively uniform distribution of the electric field.
Apoptosis analyzes
Cells were cultured in 25 cm2 Petri dishes, after 24 h flavonoids were added in IC50 concentrations (hesperetin IC50:115 μM; Quercetin IC50:200 μM) which are used in viability test. After 96 h cells were detached with 0.25% Tripsin-EDTA solution. MCF-7 cell apoptosis was analysed using a flow cytometer with Annexin V-FITC (fluorescein isothiocyanate) and PI (Propidium iodide) staining kit (BD Biosciences) to distinguish early apoptotic from necrotic cells as previously described.
Statistical analysis
SPSS (v. 20.0 Chicago, IL) was used for statistical analyses. All the data were expressed as Mean ± SD. First of all, the data were tested for homogeneity of variances. Significant time/dose interactions were found for variables and subgroups were analyzed further by testing the effect of concentration within each group using for repeated-measures ANOVA. Accordingly time/dose interactions was found significantly (P<0.05). Therefore, differences between the groups were evaluated with one-way analysis of variance followed by the Duncan test in each time groups and the difference between times was analyzed separately by paired t test in each groups.
Results
Effect of quercetin and hesperetin on cell proliferation and apoptosis
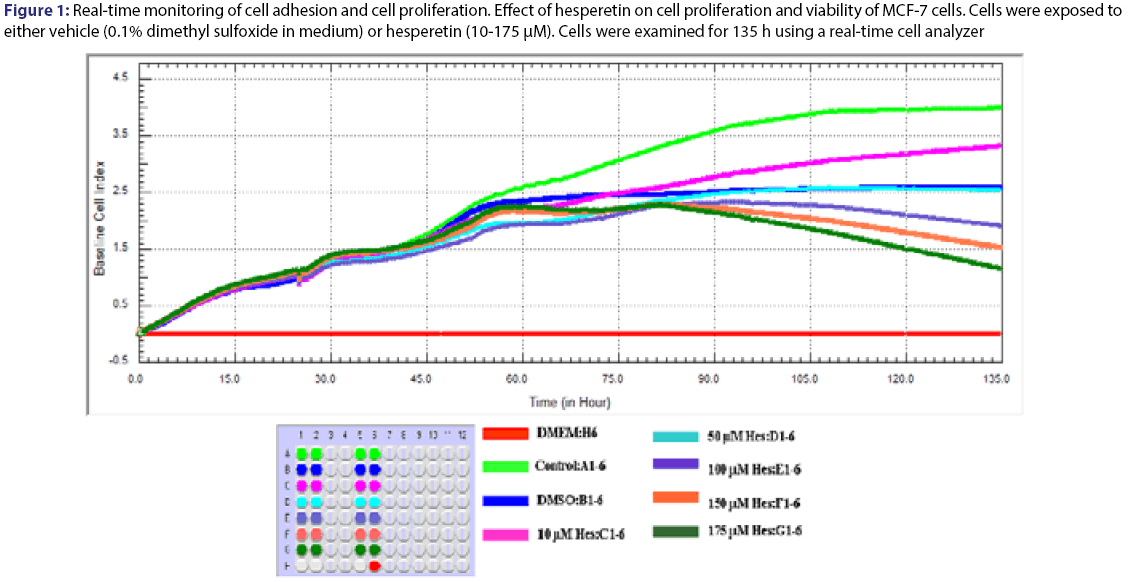
As a result of the analysis, time/dose interaction was found to be significant and therefore the comparisons between dose groups were made for each period of time. Cell growth rate was monitored for 135 hours with various concentration of hesperetin. The cells were inhibited by different concentration (10-175 μM) of hesperetin compared to control [Figure 1]. Statistical results were given in the Table 1 for effect of hesperetin on cells proliferation. A statistically difference was not found significantly (P>0.05) between dose groups after the treatment for 24 h and 48 h. Differences of between dose groups significantly were observed statistically after the treatment for 72 h [Table 1]. The treatment of MCF-7 cells with hesperetin for 48 h did not affect cell proliferation [Figure 1]. Hesperetin significantly decreased cell proliferation at 96 h of treatment in a dose- and timedependent manner (p=0.026). Inhibition of cell proliferation was seen hesperetin treatment at concentrations of 150 and 175 μM (decreased up to 50.6% and 56.9% at 96 h and decreased up to 52.8% and 60.6% at 120 h compared to the control, respectively (P<0.05) [Table 2]. IC50 dose of hesperetin (IC50: 115 μM) calculated at 96 hours (r2:0.99) and apoptosis analyze was set up by using calculated dose of hesperetin.
Figure 1: Real-time monitoring of cell adhesion and cell proliferation. Effect of hesperetin on cell proliferation and viability of MCF-7 cells. Cells were exposed to either vehicle (0.1% dimethyl sulfoxide in medium) or hesperetin (10-175 μM). Cells were examined for 135 h using a real-time cell analyzer
| 96 h | 120 h | 135 h | ||||
|---|---|---|---|---|---|---|
| CONTROL | 3.62±0.52 | a | 3.76±0.55 | a | 3.83±0.30 | a |
| DMSO | 2.21±0.04 | b | 2.28±0.11 | bc | 2.24±0.14 | c |
| 10 µM Hes | 2.43±0.58 | b | 2.76±0.67 | b | 2.84±0.42 | b |
| 50 µM Hes | 2.19±0.52 | b | 2.24±0.47 | bc | 2.19±0.21 | c |
| 100 µM Hes | 1.88±0.44 | bc | 1.75±0.37 | c | 1.68±0.13 | d |
| 150 µM Hes | 1.79±0.42 | c | 1.62±0.35 | cd | 1.52±0.30 | d |
| 175 µM Hes | 1.71±0.42 | c | 1.48±0.26 | d | 0.76±0.14 | e |
| P | 0.026 | 0.001 | 0.000 |
MCF 7 cells were treated with the indicated concentrations of hesperetin (Hes). Cell proliferation and viability were measured using a real time cell analyzer. Data are presented as mean ± SD. Groups with different superscript letters in each column represent significant difference between groups (P < 0.05).
Table 1: Cell index as mean ± standard deviation (M ± SD) on MCF-7 cells
| 96 h | 120 h | |||
|---|---|---|---|---|
| Cell index | Cell via.(%) | Cell index | Cell via.(%) | |
| CONTROL | 3.62±0.52 | 100 | 3.76±0.55 | 100 |
| 10 µM Hes | 2.43±0.58 | 67.1 | 2.76±0.67 | 73.4 |
| 50 µM Hes | 2.19±0.52 | 60.5 | 2.24±0.47 | 59.6 |
| 100 µM Hes | 1.88±0.44 | 51.9 | 1.75±0.37 | 46.5 |
| 150 µM Hes | 1.79±0.42 | 49.4 | 1.62±0.35 | 43.1 |
| 175 µM Hes | 1.71±0.42 | 47.2 | 1.48±0.26 | 39.4 |
All data are reported as the percentage change in comparison with the vehicle-only group. which were arbitrarily assigned 100% viability.
Table 2: Cell index as mean ± standard deviation (M ± SD) and percentages of cell viability
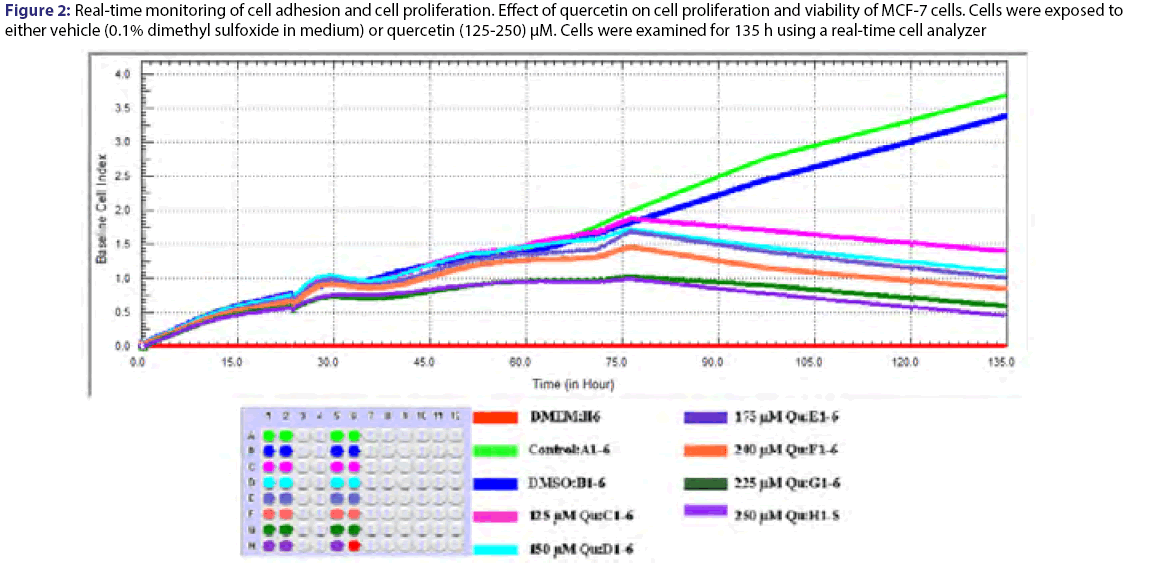
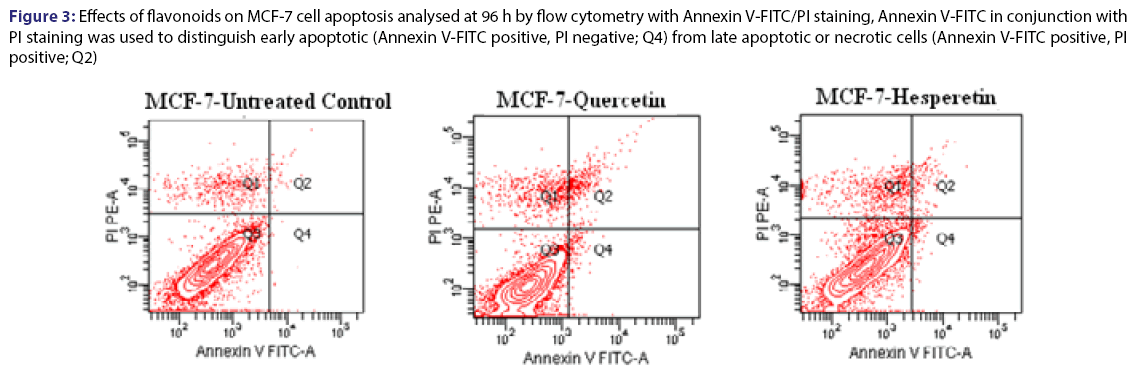
Cell proliferation rates were observed various concentrations of quercetin at 135 h. The cells were inhibited by different concentration (125-250) μM of quercetin compared to control [Figure 2]. Statistical results were given in the Table 3 for effect of quercetin on cells. The dose groups were found different compared to control, except 225 and 250 μM after the treatment for 24 h. It was seen a similar situation in terms of statistical significance at next times, but the degree of differences between dose groups were changed from time to time due to interaction time/dose [Table 3]. The treatment of MCF-7 cells with quercetin after the treatment for 48 h did not affect cell proliferation [Figure 2]. Quercetin significantly decreased cell proliferation after the treatment for 72 h of treatment in a dose-and time-dependent manner (P<0.05). Inhibition of cell proliferation was seen quercetin treatment at concentrations of 200 and 250 μM (decreased up to 57.4% and 68.5% at 96 h and decreased up to 70.4% and 81.1% at 120 h compared to the control, respectively P<0.05) [Table 4]. IC50 dose of quercetin (IC50: 200 μM) calculated at 96 hours (r2:0.99) and apoptosis analyze was set up by using calculated dose of quercetin. As a result of the analysis, time/dose interaction was found to be significant and therefore the comparisons between dose groups were made separately for each period of time. It is observed that flavonoids induce apoptosis in MCF-7 cells at 96 hours as well as cell viability [Figure 3]. Apoptosis was in an increasing trend: it was 9-fold for hesperetin and 13.7-fold for Quercetin (Annexin V positive, PI negative) at 96 hours [Table 5].
Figure 2: Real-time monitoring of cell adhesion and cell proliferation. Effect of quercetin on cell proliferation and viability of MCF-7 cells. Cells were exposed to either vehicle (0.1% dimethyl sulfoxide in medium) or quercetin (125-250) μM. Cells were examined for 135 h using a real-time cell analyzer
Figure 3: Effects of flavonoids on MCF-7 cell apoptosis analysed at 96 h by flow cytometry with Annexin V-FITC/PI staining, Annexin V-FITC in conjunction with PI staining was used to distinguish early apoptotic (Annexin V-FITC positive, PI negative; Q4) from late apoptotic or necrotic cells (Annexin V-FITC positive, PI positive; Q2)
| 72 h | 96 h | 120 h | 135 h | |||||
|---|---|---|---|---|---|---|---|---|
| CONTROL | 1.71±0.44 | a | 2.70±0.64 | a | 3.27±0.76 | a | 3.70±0.64 | a |
| DMSO | 1.71±0.01 | a | 2.53±0.22 | a | 3.01±0.28 | a | 3.48±0.22 | a |
| 125 µM Qu | 1.81±0.28 | a | 1.64±0.24 | b | 1.52±0.46 | b | 1.42±0.17 | b |
| 150 µM Qu | 1.80±0.21 | a | 1.45±0.11 | bc | 1.32±0.14 | bc | 1.20±0.03 | bc |
| 175 µM Qu | 1.79±0.03 | a | 1.42±0.05 | bc | 1.30±0.09 | cd | 1.17±0.10 | bc |
| 200 µM Qu | 1.52±0.06 | ab | 1.15±0.08 | bcd | 1.03±0.19 | cd | 0.92±0.08 | bcd |
| 225 µM Qu | 1.05±0.22 | b | 0.95±0.16 | cd | 0.79±0.18 | de | 0.63±0.09 | cd |
| 250 µM Qu | 1.01±0.22 | b | 0.80±0.17 | d | 0.62±0.21 | e | 0.43±0.12 | d |
| P | 0.002 | 0.001 | 0.000 | 0.000 |
MCF 7 cells were treated with the indicated concentrations of quercetin (Qu). Cell proliferation and viability were measured using a real time cell analyzer. Data are presented as mean ± SD. Groups with different superscript letters in each column represent significant difference between groups (P < 0.05).
Table 3: Cell index as mean ± standard deviation (M ± SD) on the MCF-7 cells
| 72 h | 96 h | 120 h | ||||
|---|---|---|---|---|---|---|
| Cell index | Cell via.(%) | Cell index | Cell via.(%) | Cell index | Cell via.(%) | |
| CONTROL | 1.71±0.44 | 100 | 2.70±0.64 | 100 | 3.27±0.76 | 100 |
| 125 µM Qu | 1.81±0.28 | 105.8 | 1.64±0.24 | 60.7 | 1.52±0.46 | 46.5 |
| 150 µM Qu | 1.80±0.21 | 105.3 | 1.45±0.11 | 53.7 | 1.32±0.14 | 40.4 |
| 175 µM Qu | 1.79±0.03 | 104.7 | 1.42±0.05 | 52.6 | 1.30±0.09 | 39.8 |
| 200 µM Qu | 1.52±0.06 | 88.9 | 1.15±0.08 | 42.6 | 1.03±0.19 | 31.5 |
| 225 µM Qu | 1.05±0.22 | 61.4 | 0.95±0.16 | 35.2 | 0.79±0.18 | 24.2 |
| 250 µM Qu | 1.01±0.22 | 59.0 | 0.80±0.17 | 29.6 | 0.62±0.21 | 18.9 |
All data are reported as the percentage change in comparison with the vehicle-only group. which were arbitrarily assigned 100% viability.
Table 4: Cell index as mean ± standard deviation (M ± SD) and percentages of cell viability
| Annexin V(-) PI(-)(%) |
Annexin V(-) PI(+)(%) |
Annexin V(-) PI(+)(%) |
||
|---|---|---|---|---|
| Control (No treatment) | 92.3 | 0.3 | 0.5 | |
| Quercetin (200 µM) | 86.1 | 4.1 | 2.1 | |
| Hesperetin (115 µM) | 81.1 | 2.7 | 2 | |
Apoptosis analyzes of MCF-7 after flavonoids treatment at 96 h. Early apoptotic (Annexin V(-)/PI(+)); late apoptotic Annexin V(-)/PI(+); live cells Annexin V(-)/ PI(-)
Table 5: Apoptosis values of MCF-7 after flavonoids treatment
Discussion and Conclusion
Flavonoids are generally known as inhibitors of cell proliferation. Several recent studies have shown anti-proliferative effects of flavonoid compounds on cancer cells. Studies have been emphasized to reduce risk of cancer if major food sources of these compounds were consumed frequently.[3,8,9,11,20,27-30] Anti-proliferative effects of flavonoids on cancer cells were described by several mechanisms. These compounds were interested that may inhibit cell cycle or induce apoptosis.[3,8] Braganhol et al. analyzed effects of quercetin on human glioma cells (U138MG). The cells were treated with 10-100 μM of quercetin and were analyzed at 24, 48 and 72 h after treatment by MTT analyze. The cells proliferation reduced with 30 μM of quercetin at these times for 22, 58, 74%, respectively, but these rates changed with 100 μM as 31, 70, 76%. Quercetin caused G2/M phase arrest by increasing the caspase 3 and caspase 7 in this study.[31] Chou et al. Studied effect of quercetin on MCF-7 cells and were treated with different dose (10-175 μM) of quercetin. Effect of quercetin was analyzed at 24 and 48 h and inhibition of cells was observed in a dose-time dependent manner by MTT analyze.[27] Also the cell cycle was analyzed and numbers of cells were found to decrease significantly in G0/G1 phase. Quercetin caused S phase arrest by decreasing the protein expression of CDK2, cyclins A and B.[24] Alshatwi et al. investigated effects of hesperetin on cell viability in human cervical cancer cells (SiHa). SiHa cells were exposed to various concentrations of hesperetin (0-1000 μM) for 24 and 48 h and were measured by MTT assay. According to this study, hesperetin did not inhibit the cells at lower concentrations, but cell viability reduced at a hesperetin concentration of 500 μM after 24 and 48 h of the treatment. Hesperetin significantly inhibited the cells in a time-and dose-dependent manner at the high concentration (1000 μM).[32] In another study, Choi et al. searched anticancer effect of hesperetin on MCF-7 cells. Cell proliferation was inhibited by hesperetin induced G1- phase cell cycle arrest on MCF-7 cells. Also cyclin dependent kinases was examined in this context. Effect 10, 50 and 100 μM of hesperetin was analyzed on MCF-7 at 24, 48 and 72 h by MTT assay. They have been observed to inhibit the cell proliferation a dose-time dependent manner. Accordingly, maximum inhibition was seen at 72 h with 100 μM hesperetin.[8] In our study, proliferation of cells was inhibited in a time- and dose-dependent manner by flavonoids. MCF-7 cells were treated with different doses (125-250 μM) of quercetin and (10-175 μM) hesperetin. This effect was performed by a real-time cell analyzer. Inhibitory effect of these flavonoids was observed by MTT after the treatment for 24 h by according to the mentioned studies, but we significantly observed effect of these flavonoids after the treatment for 72 h and subsequent times with a real-time cell analyzer. Hesperetin and Quercetin shows specific inhibitory activity for cancer cell growth,[15,16] but the mechanisms underlying the effects of hesperetin and quercetin in the induction of cell cycle arrest in human breast cancer cells are still unknown.
According to our results, 115 μM for hesperetin and 200 μM for quercetin may useful for treatment of breast cancer in later times. Furthermore the flavonoids induced apoptosis of cells. Flavonoids inhibit the cell proliferation via induce cell apoptosis. Flavonoids may have shown inhibitor effects on cell proliferation by increasing Bax or decreasing Bcl-2 or effecting caspase activation, which are effective in apoptosis process. Moreover Quercetin was effective at earlier times and at higher concentrations than hesperetin on inhibition of MCF-7 proliferation. It may be a reason that quercetin have two more hydroxyl groups, unlike hesperetin. Because hydroxyl groups which are found flavonoids provide antoxidant or antiproliferative characteristic property of them.
Our results are generally consistent with this literature in terms of to inhibit the cell proliferation a dose-time dependent manner. The difference between the results could be attributable to methodology and different application concentrations. Classical viability assays are both time-consuming and non-sensitive methods. Furthermore the specifity of methods is low and quite obvious to the mistakes of manipulation. In the present work, we conducted experiments with a new real-time system that investigated the effects of the hesperetin and quercetin on MCF-7 cell line by real-time and continuous monitoring of cell proliferation and viability. Furthermore, the real-time cell analysis system allowed for calculation of time-dependent different concentrations.[33,34] Compared with conventional end point cell-based assays, dynamic monitoring of cell response, such as cell adhesion, spreading, proliferation, and cell death, is an advantage of the real-time system to optimize the cell concentration for in-vitro assays; it also allows both cell and assay conditions to be constantly obtained before and during the experiments.[35]
Conflict of interest
The authors declare no conflict of interest.
References
- IARC. Fruit and Vegetables France2003.
- Ben SM, Skandrani I, Nasr N, Franca MG, Chekir-Ghedira L, Ghedira K. Flavonoids and sesquiterpenes from Tecuriumramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: a structure-activity relationship study. Environmental toxicology and pharmacology 2011;32:336-48.
- So FV, Guthrie N, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer letters 1997;112:127-33.
- Cooray HC, Janvilisri T, van VHW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochemical and biophysical research communications 2004;317:269-75.
- Kizilkeçili Ö. Determining antifungal, antibacterial and anti-tuberculosis activity of methanol, ethanol extracts and volatile oils of salvia crypthantamontbret&auchr ex benthamve salvia pomifera L. species. Department of Biology Turkey: Balikesir; 2007.
- Ullah MF, Ahmad A, Zubair H. Soy isoflavonegenistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Molecular nutrition & food research 2011;55:553-9.
- Gibellini L, Pinti M, Nasi M. Quercetin and cancer chemoprevention. Evidence-based complementary and alternative medicine: eCAM 2011;2011:591356.
- Choi EJ. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: involvement of CDK4 and p21. Nutrition and cancer 2007;59:115-9.
- Choi EJ, Bae SM, Ahn WS. Anti-proliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Archives of pharmacal research 2008;31:1281-5.
- Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanonesnaringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. The Journal of nutrition 2001;131:235-41.
- Kim JY, Jung KJ, Choi JS, Chung HY. Hesperetin: a potent antioxidant against peroxynitrite. Free radical research 2004;38:761-9.
- Lee KS, Kim EY, Jeon K.3,4-Dihydroxyflavone acts as an antioxidant and anti-apoptotic agent to support bovine embryo development in vitro. The Journal of reproduction and development 2011;57:127-34.
- Michaud-Levesque J, Bousquet-Gagnon N, Beliveau R. Quercetin abrogates IL-6/STAT3 signalling and inhibits glioblastoma cell line growth and migration. Experimental cell research 2012;318:925-35.
- Shimoda K, Hamada H, Hamada H. Glycosylation of hesperetin by plant cell cultures. Phytochemistry 2008;69:1135-40.
- Garcia-Lafuente A, Guillamon E, Villares A, Rostagno MA, Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflammation research: official journal of the European Histamine Research Society2009;58:537-52.
- Beliveau R, Gingras D. Role of nutrition in preventing cancer. Canadian family physician Medecin de famillecanadien 2007;53:1905-11.
- Guha N. Dietary flavonoids and the risk of breast cancer and childhood leukemia. Berkeley: California University. 2009;135.
- Nalini N, Aranganathan S, Kabalimurthy J. Chemopreventive efficacy of hesperetin (citrus flavonone) against 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Toxicology mechanisms and methods 2012;22:397-408.
- Liu KC, Yen CY, Wu RS. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environmental toxicology 2014;29:428-39.
- Ye L, Chan FL, Chen S, Leung LK. The citrus flavonone hesperetin inhibits growth of aromatase-expressing MCF-7 tumor in ovariectomizedathymic mice. The Journal of nutritional biochemistry 2012;23:1230-7.
- Zarebczan B, Pinchot SN, Kunnimalaiyaan M, Chen H. Hesperetin, a potential therapy for carcinoid cancer. American journal of surgery 2011;201:329-33.
- Kocaba? N. Protective effect of quercetin on homocysteine-induced oxidative stress. Department of biochemistry. Turkey: AfyonkarahisarKocatepe 2008.
- Atienza JM, Zhu J, Wang X, Xu X, Abassi Y. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. Journal of bio-molecular screening 2005;10:795-805.
- Atienza JM, Yu N, Wang X, Xu X, Abassi Y. Label-free and real-time cell-based kinase assay for screening selective and potent receptor tyrosine kinase inhibitors using microelectronic sensor array. Journal of bio-molecular screening 2006;11:634-43.
- Yu N, Atienza JM, Bernard J. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: an approach to study G protein-coupled receptors. Analytical chemistry 2006;78:35-43.
- Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods in molecular biology (Clifton, NJ) 2011;740:33-43.
- Chou CC, Yang JS, Lu HF. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Archives of pharmacal research 2010;33:1181-91.
- Cutler GJ, Nettleton JA, Ross JA. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa Women's Health Study. International journal of cancer Journal international du cancer 2008;123:664-71.
- Rossi M, Lugo A, Lagiou P. Proanthocyanidins and other flavonoids in relation to pancreatic cancer: a case-control study in Italy. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 2012;23:1488-93.
- Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. The American journal of clinical nutrition 2009;89:905-12.
- Braganhol E, Zamin LL, Canedo AD. Anti-proliferative effect of quercetin in the human U138MG glioma cell line. Anti-cancer drugs 2006;17:663-71.
- Alshatwi AA, Ramesh E, Periasamy VS, Subash-Babu P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundamental & clinical pharmacology 2013;27:581-92.
- Ozturk F, Malkoc S, Ersoz M, Hakki SS, Bozkurt BS. Real-time cell analysis of the cytotoxicity of the components of orthodontic acrylic materials on gingival fibroblasts. American journal of orthodontics and dentofacial orthopaedics: official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics 2011;140:e243-9.
- Urcan E, Haertel U, Styllou M, Hickel R, Scherthan H, Reichl FX. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dental materials: official publication of the Academy of Dental Materials 2010;26:51-8.
- Reichl FX, Esters M, Simon S. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Archives of toxicology 2006;80:370-7.