Effect of Potentilla fulgens on lipid peroxidation and antioxidant status in alloxan-induced diabetic mice
- *Corresponding Author:
- Donkupar Syiem
Department of Biochemistry, North Eastern Hill University, Shillong, Meghalaya, India.
E-mail: dsyiem@yahoo.com
Date of Received: 26-03-2012
Date of Accepted: 26-04-2012
Available Online: 15-05-2012
Abstract
Potentilla fulgens (Rosaceae) root traditionally used as a folk remedy by local health practitioners of Khasi Hills, Meghalaya was investigated for its effects on lipid peroxidation and antioxidant status in alloxan-induced diabetic mice. Significant increase in levels of thiobarbituric acid reactive substances (TBARS) and decrease in activities of glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) were observed under diabetic condition. Intraperitoneal administration of methanol extract of P. fulgens roots at a dose of 250 mg/kg body weight to male swiss albino diabetic mice for 14 days caused significant reduction in the elevated TBARS level, while increasing the activities of the antioxidant enzymes in diabetic mice. Maximum reduction in TBARS level was observed in liver tissue (75%, p<0.001). Kidney exhibited the highest elevation in the activity for catalase (68%, p<0.001) and superoxide dismutase (29%, p<0.001) while maximum increase in glutathione peroxidase activity was seen in brain (50%, p<0.001). The effects of P. fulgens was compared against known antioxidant, vitamin C. Results indicate that Potentilla fulgens methanolic root extract can reduce free radical mediated oxidative stress in experimental diabetes mellitus.
Keywords
Antioxidant, alloxan, diabetes mellitus, Potentilla fulgens
Introduction
Diabetes mellitus is a disease that has been characterized by a series of complications affecting several tissues in the body. Free radicals have been implicated in the pathophysiology of diabetes [1, 2] and oxidative stress may be a common pathway linking diverse mechanisms for the complications in diabetes [3]. High blood glucose concentrations leading to hyperglycemia cause free radicals to be produced via glucose autoxidation [4], non-enzymatic protein glycation [5], increased influx toward polyol pathway [6], activation of protein kinase C and increased flux through hexosamine pathway [7]. There is evidence that diabetes alters free radical metabolism in blood and tissues [8-10]. Cellular damage by cytotoxic oxygen free radical species leads to complications in diabetes and must, therefore, be rapidly and efficiently scavenged if cellular damage is to be prevented [11, 12]. Degenerative changes in tissues leading to cardiomyopathy, nephropathy and neuropathy are common complications of diabetes mellitus [11]. In addition, significant changes in lipid metabolism and structure also occur in diabetes particularly in patients with vascular complications and atherosclerosis [13-15]. Abnormally high levels of free radicals and decline of antioxidant defense mechanisms lead to damage of cellular organelles and enzymes, increased lipid peroxidation, and development of insulin resistance [16].
The protection of cells from oxidative stress is essential and under normal conditions adequate protection is provided by the antioxidant systems. Mammalian cells possess elaborate defense mechanisms for free radical detoxification involving metabolic enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) [17]. Non-enzymatic molecules, including thioredoxin, thiols, vitamins E, C and trace metals, such as selenium function as direct scavengers of ROS [18].
In recent years, interest in finding naturally occurring antioxidants that can protect against oxidative stress has increased considerably [19], with a view to their use in foods or as medicinal formulations to replace synthetic antioxidants which are being restricted due to their side effects such as carcinogenicity [20]. Natural antioxidants have been reported to have a wide range of biochemical activities, including inhibition of ROS generation, direct or indirect scavenging of free radicals, and alteration of intracellular redox potential [21, 22]. The use of plants as source of drugs and their validation is gaining increasing attention [23, 24].
Potentilla fulgens L. of the Rosaceae family, commonly found at higher altitudes (1500-2000 m MSL) of Khasi Hills, Meghalaya, India has been used as folk remedy. Traditionally, pieces of roots are chewed along with betel, composed of raw areca nut (Areca catechu), locally called Kwai, and betel leaf (Piper betel). It has been shown that roots of Potentilla fulgens possess hypoglycemic and anti-hyperglycemic properties in mice [25], and also affect the polyol pathway [26]. We have also reported the antioxidant activity of P. fulgens using in-vitro systems [27]. Recently, Jaitak et al [28] reported the presence of antioxidant compounds epicatechin and a new biflavanoid (Potifulgene) in P. fulgens. Studies on other species of potentilla have also been reported and reviewed [29]. This study was undertaken to further investigate the effect of methanol extract of P. fulgens on the lipid peroxidation and activities of the antioxidant enzymes, catalase, superoxide dismutase and glutathione peroxidase in alloxan induced diabetic mice and to compare its effects with that of a standard antioxidant, vitamin C.
Materials and Methods
Chemicals
Alloxan monohydrate, thiobarbituric acid, glutathione reduced, 5,5- dithiobis-(2-nitrobenzoic acid (DTNB), 1,1,3,3-tetramethoxypropane (TMOP), were procured from Sigma-Aldrich (St. Louis, USA) and ascorbic acid, pyrogallol, sodium azide, Diethylenetriaminepentaacetic acid (DETAPAAC), sodium lauryl sulphate, bovine serum albumin were purchased from Sisco Research laboratories. Other chemicals used were of analytical grade obtained from SRL, Hi-media and Rankem, India.
Plant material
P. fulgens was collected from Shillong peak area of Meghalaya and the plant specimen (voucher no. 464) was submitted and identified by herbarium curator Dr. P.B. Gurung, Department of Botany, NEHU, Shillong.
Plant extraction
The roots of P. fulgens were washed, shredded and dried in the shade and then powdered using a grinder. The dried powder was repeatedly extracted with 10 volumes of aqueous-methanol solution (1:4) [30]. The mixture was filtered and the filtrate was dried under vacuum in a rotary evaporator (Yamato RE800) yielding the crude methanol extract. The yield of the methanol extract (w/w from dried starting material) was 7.76 %.
Experimental Animals
Healthy, Swiss albino mice of approximately 6 months were used for the study. Mice were housed in a room kept under controlled conditions with temperature maintained at 22°C on a 12-h light: 12-h dark cycle and were fed with balanced mice feed obtained from Amrut Laboratory, Pune, India. Institutional ethical guidelines were followed for all experiments.
Preparation of diabetic mice
Animals were administered alloxan monohydrate prepared in acetate buffer (0.15 M, pH 4.5) via intraperitonal (i.p.) route [25]. Prior to administration, mice were fasted over night but given water ad libitum. Mice with more than 3-4 fold increased in blood glucose (measured using glucostix; SDCheck) were considered diabetic.
Experimental design
In this study, mice were divided into 4 groups (comprising 6 animals in each group). Two groups of alloxan-induced diabetic mice were administered the extract (250 mg/kg b.w.) and vitamin C (250mg/kg b.w.) respectively, on alternate days via i.p. route for a period of 14 days.
Group 1: Normal untreated mice
Group 2: Diabetic untreated mice
Group 3: Diabetic mice treated with extract (250 mg/kg b.w.)
Group 4: Diabetic mice treated with Vitamin C (250mg/kg b.w.)
At the end of 14 days, experimental animals were sacrificed by cervical dislocation and dissected carefully to remove the liver, kidney, brain and heart of individual group for subsequent biochemical analysis.
Lipid peroxidation assay
Lipid peroxidation was estimated by measurement of thiobarbituric acidreactive substances (TBARS) according to the method of Okhawa et al [31]. The pink chromogen produced by the reaction of thiobarbituric acid with malondialdehyde (MDA), a secondary product of lipid peroxidation was measured at λ 532 nm. The tissues were weighed and homogenized (10% w/v) in ice-cold 1.15% KCl and centrifuged at 1000 x g for 10 mins at 4ºC. The pellet was discarded and supernatant was used to determine the TBARS levels. TMOP was used as the standard, and the level of lipid peroxides was expressed as μmoles of MDA.
Assays for antioxidant enzymes
Tissues were weighed and homogenized (10% w/v) in 10 mM potassium phosphate buffer, pH 7.0, and centrifuged at 18000 x g for 15 min at 4ºC. The pellet was discarded and the supernatant was used as enzyme preparations. Protein concentrations of the tissue homogenates were determined according to the method of Bradford using bovine serum albumin (BSA) as the standard [32].
Catalase (CAT) activity was measured according to the method of Aebi [33] by determining the rate of degradation of hydrogen peroxide at λ 240 nm. The extinction co-efficient of 71 mM-1cm-1 was used for calculation. One unit is defined as 1 mmol of H2O2 consumed/minute and the specific activity is reported as units/ mg protein
The activity of superoxide dismutase (SOD) was assayed by the method of Marklund and Marklund [34]. The degree of inhibition of autoxidation of pyrogallol, in an alkaline pH by SOD was used as a measure of enzyme activity by measuring the change in absorbance at λ 470 nm. Enzyme activity is defined as the amount of enzyme required for 50% inhibition of pyrogallol autoxidation /min (units/ mg protein).
Glutathione peroxidase (GPx) was assayed by measuring the amount of reduced glutathione (GSH) at λ 412 nm according to the method of Rotruck et al [35]. The activity of GPx is expressed as μmoles of GSH utilized/min/mg protein.
Statistical Analysis
Student’s ‘t’-test was used for determining the levels of significance between the normal, diabetic control and the diabetic treated groups. Results are expressed as mean ± standard error of mean (S.E.M.)
Results
Effect on Lipid Peroxidation
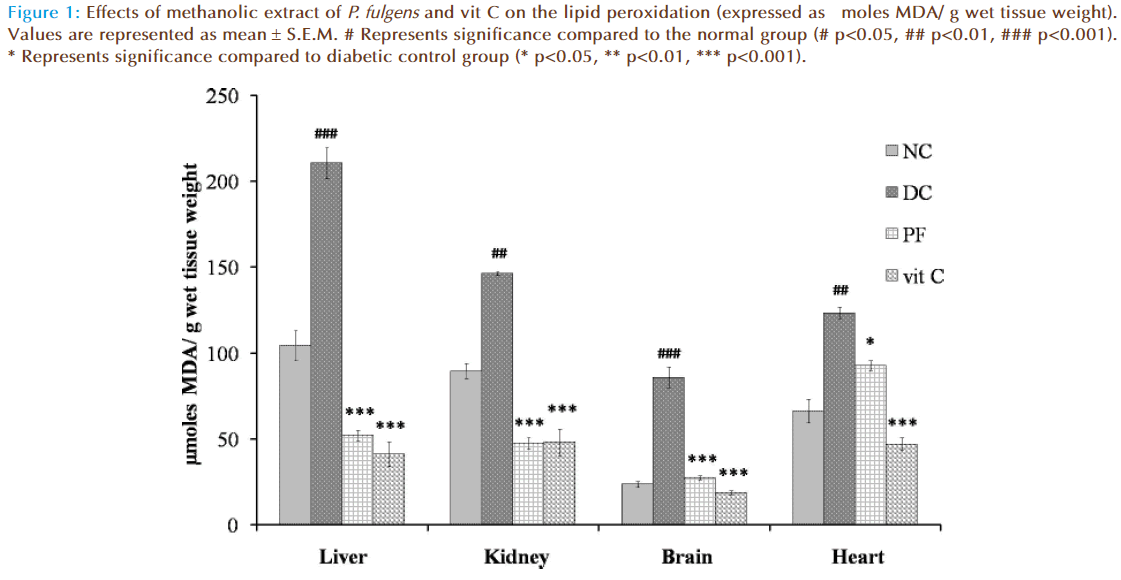
Figure 1 shows the effect of P. fulgens extract and vit C on tissue lipid peroxidation in diabetic test groups. Significant increase in TBARS level was seen in untreated diabetic group compared to the normal group (104.43 ± 8.5 μmoles MDA in liver, 89.43 ± 9.01 in kidney, 23.66 ± 3.08 in brain and 66.268 ± 7.1 in heart). TBARS level in liver was increased by 101.98% (p<0.001), 63.53% (p<0.01) in kidney, 262.63% (p<0.001) in brain and 86% (p<0.01) in heart tissues as compared to the normal group. Lipid peroxidation in diabetic mice treated with P. fulgens extract and vit C was significantly reduced. Thus, P. fulgens reduced TBARS level by 75.21% (p<0.001) in liver, 67.5% (p<0.001) in kidney, 67.93% (p<0.001) in brain and 24% (p<0.05) in heart from the diabetic control group. Treatment of vit C decreased lipid peroxidation level by 80% (p<0.001) in liver, 67.1% (p<0.001) in kidney, 78% (p<0.001) in brain and 61.9% (p<0.001) in heart.
Figure 1: Effects of methanolic extract of P. fulgens and vit C on the lipid peroxidation (expressed as μmoles MDA/ g wet tissue weight). Values are represented as mean ± S.E.M. # Represents significance compared to the normal group (# p<0.05, ## p<0.01, ### p<0.001). * Represents significance compared to diabetic control group (* p<0.05, ** p<0.01, *** p<0.001).
Effect on Antioxidant Enzymes
Catalase
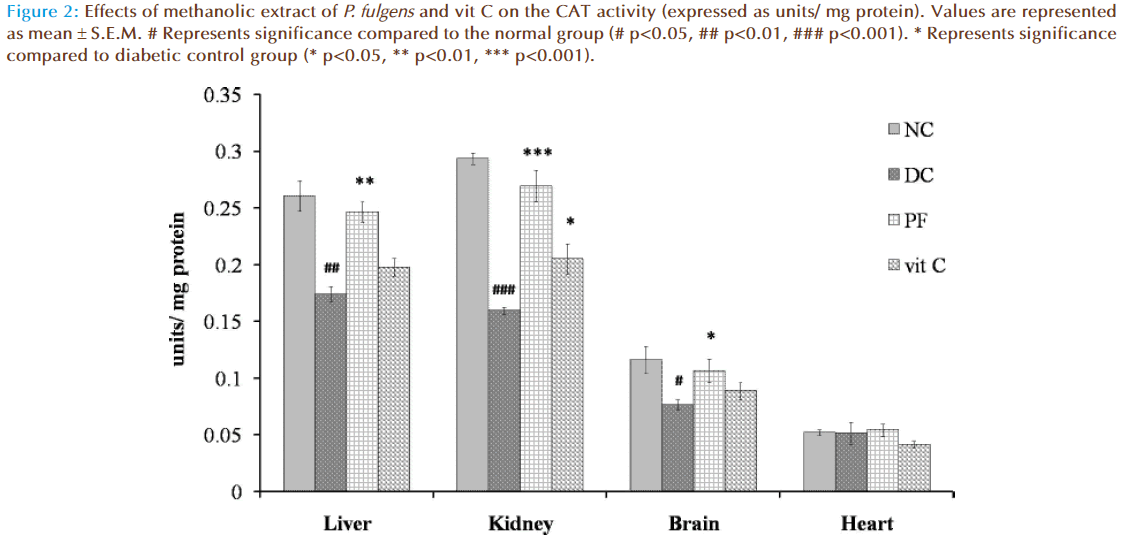
Figure 2 shows the effect of P. fulgens extract and vit C on CAT activity in diabetic test groups. Significant decrease in CAT activity was observed in liver, kidney and brain tissues of untreated diabetic group compared to the normal levels (0.26 ± 0.023 units/ mg protein in liver, 0.29 ± 0.008 in kidney, 0.12 ± 0.012 in brain and 0.05 ± 0.002 in heart). CAT activity in liver was reduced by 33% (p<0.01), in kidney by 45% (p<0.001) and in brain by 34% (p<0.05) as compared to the normal group. Administration of P. fulgens extract increased CAT activity by 41% (p<0.01) in liver, 68% (p<0.001) in kidney, 39% (p<0.05) in brain from the diabetic control group. Effect of treatment with vit C was significant only on kidney tissue with an increase in CAT activity by 28% (p<0.05) from the diabetic control mice. No significant change of CAT activity was observed in heart tissues of both untreated and treated diabetic groups.
Figure 2: Effects of methanolic extract of P. fulgens and vit C on the CAT activity (expressed as units/ mg protein). Values are represented as mean ± S.E.M. # Represents significance compared to the normal group (# p<0.05, ## p<0.01, ### p<0.001). * Represents significance compared to diabetic control group (* p<0.05, ** p<0.01, *** p<0.001).
Superoxide dismutase
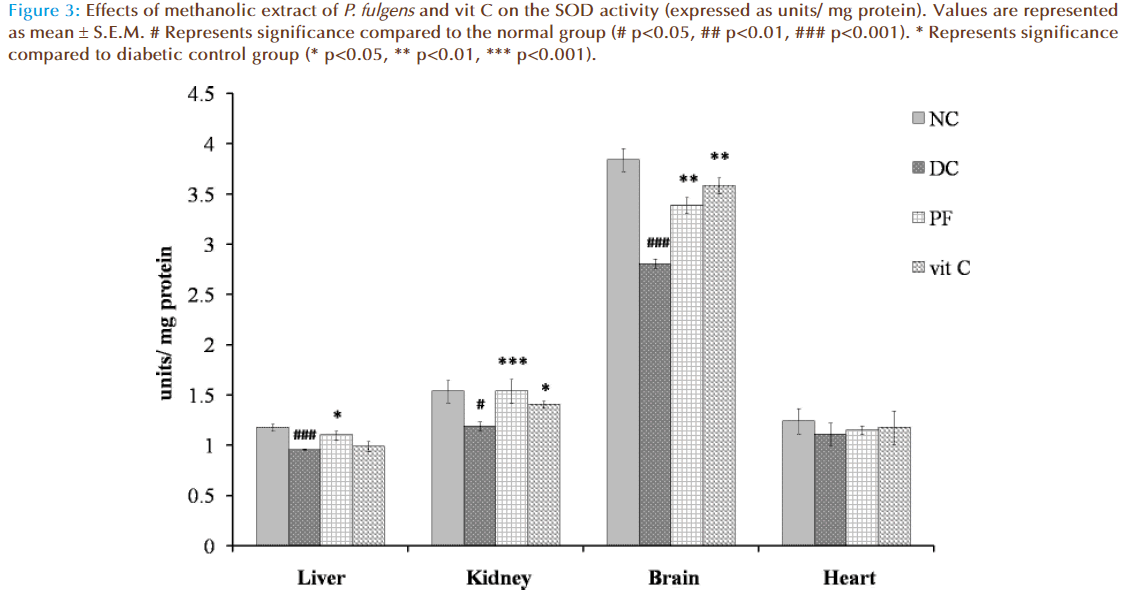
Figure 3 shows the effect of P. fulgens extract and vit C on SOD activity in diabetic test groups. Significant decrease in SOD activity was observed in liver, kidney and brain tissues of untreated diabetic group compared to the normal levels (1.175 ± 0.036 units/ mg protein in liver, 1.533 ± 0.11 in kidney, 3.82 ± 0.11 in brain and 1.24 ± 0.12 in heart). SOD activity was reduced by 18% (p<0.01), 22% (p<0.05) and by 26% (p<0.001) in liver, kidney and brain, respectively, as compared to the normal group. Administration of P. fulgens extract increased enzyme activity by 15% (p<0.05), 29% (p<0.001), 20% (p<0.01) in liver, kidney and brain, respectively, from the diabetic control group. Treatment with vit C significantly increased SOD activity in kidney and brain by 18% (p<0.05) and 29% (p<0.01) respectively, from the diabetic control mice. SOD activity was not significantly altered in heart tissues of both untreated and treated diabetic groups.
Figure 3: Effects of methanolic extract of P. fulgens and vit C on the SOD activity (expressed as units/ mg protein). Values are represented as mean ± S.E.M. # Represents significance compared to the normal group (# p<0.05, ## p<0.01, ### p<0.001). * Represents significance compared to diabetic control group (* p<0.05, ** p<0.01, *** p<0.001).
Glutathione peroxidase
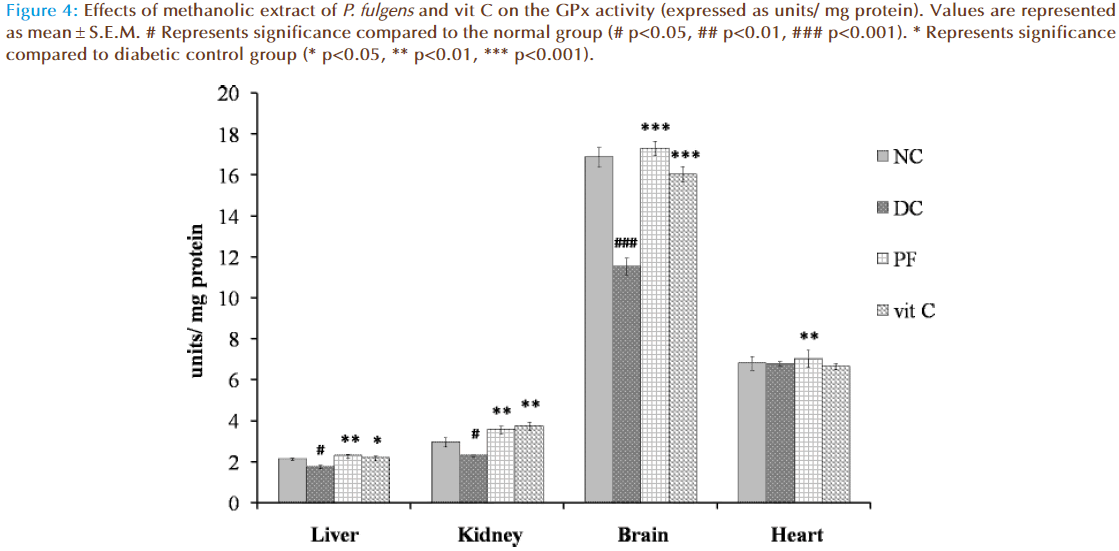
Figure 4 shows the effect of P. fulgens extract and vit C on GPx activity in diabetic test groups. Significant decrease in GPx activity was observed in liver, kidney and brain tissues of untreated diabetic group compared to the normal group (2.12 ± 0.06 units/ mg protein in liver, 2.95 ± 0.212 in kidney, 16.87 ± 0.46 in brain and 6.81 ± 0.34 in heart). GPx activity in liver was reduced by 18% (p<0.05), in kidney by 22% (p<0.05) and in brain by 31% (p<0.001) as compared to the normal group. Administration of P. fulgens extract increased GPx activity by 31% (p<0.01), 54% (p<0.01), 50% (p<0.001) in liver, kidney and brain, respectively, from the diabetic control group. Treatment with vit C significantly increased GPx activity in liver, kidney and brain by 25% (p<0.05), 62% (p<0.01) and 39% (<0.001) respectively, from the diabetic control mice. There was no significant alteration in GPx activity in heart tissues of both untreated and treated diabetic groups.
Figure 4: Effects of methanolic extract of P. fulgens and vit C on the GPx activity (expressed as units/ mg protein). Values are represented as mean ± S.E.M. # Represents significance compared to the normal group (# p<0.05, ## p<0.01, ### p<0.001). * Represents significance compared to diabetic control group (* p<0.05, ** p<0.01, *** p<0.001).
Discussion
In the present study, the antioxidant attributes of the methanolic root extract of P. fulgens were evaluated in alloxan-induced diabetic male mice. Alloxan increases oxygen free radicals production which is primarily due to hyperglycaemia [1], the effect being brought about by the destruction of pancreatic-β cells producing insulin [36].
Induction of diabetes in mice with alloxan results in an increase in thiobarbituric acid reactive substances (TBARS), an index of lipid peroxidation [16] which has been implicated in a number of adverse effects such as increased membrane rigidity, osmotic fragility, decreased cellular deformability, reduced erythrocyte survival, lipid fluidity and neurodegenerative disorders [37, 38]. MDA is one of the better known aldehydic products of lipid peroxidation, which has been the most used as a marker of oxidative stress. MDA comes from the oxidation of polyunsaturated fatty acids (PUFAs) bearing more than two double bonds such as linolenic acid, arachidonic acid, which are vulnerable to attack by free radical species [39, 40]. The observed significant elevation in TBARS level in liver, kidney, brain and heart tissues of alloxan-induced diabetic mice suggests an intensified free radical generation in diabetic mice compared to the normal mice. Treatment with P. fulgens extract at 250 mg/kg b.w. reversed TBARS buildup below the normal level observed in normal group, conferring the antioxidative and free radical quenching nature of the extract which is comparable to an equivalent dose of vit C. The effects were tissue specific.
Alterations in the tissue antioxidant enzyme activities during diabetes observed in the current study are consistent with other reports [41, 42] wherein an increase in lipid peroxides and decrease in antioxidant enzymes accompany diabetes mellitus. Kennedy and Baynes [43] suggested that decreased antioxidant enzymes activities in diabetes mellitus is due to nonenzymatic glycosylation of the enzymes. The lower activities of CAT, SOD and GPx observed in liver, kidney and brain during diabetes may be due to the inactivation or inhibition of the enzymes by the increased production of alloxan-generated reactive oxygen species (ROS) [44], while the insignificant changes in the antioxidant enzyme activities in heart could imply that some tissues are more resistant to oxidative damage than others [45]. In the P. fulgens-treated group, enzymes activities increased selectively in a tissue specific manner from that of the diabetic control. The methanol extract of P. fulgens afforded protection as evidenced by decreased TBARS and the near normal activities of these enzymatic antioxidants observed, which may be due to decreased oxidative stress. Veglioglu et al [46] established that antioxidant activity of many compounds of botanical origin is proportional to the total phenolic content (TPC), thereby suggesting a causative relationship between TPC and antioxidant activity. Incidentally, we can say that the high TPC of methanol extract of P. fulgens [27] together with the synergistic action of other bioactive principles in the extract may have contributed to its protective effects against oxidative stress-mediated diabetic complications.
Conclusion
The present study demonstrated that the methanol extract of Potentilla fulgens exerted significant antioxidant potential in diabetes-induced oxidative stress and therefore merits further investigation in terms of its active constituents and mechanism of action.
Acknowledgment
We thank UGC funded UPE program for NEHU, DBT, DST through FIST and the Department of Biochemistry, North-Eastern Hill University, Shillong, India for providing facilities.
References
- Oberley LW. Free radicals and diabetes. Free Radical Biology Medicine. 1988; 5: 113-124.
- Brownlee M, Cerami A. The biochemistry of the complications of diabetes. Annual Review of Biochemistry. 1981; 50: 385-432.
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991; 40: 405-412.
- Hunt JV, Smith CC, Wolff SP. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by lucose. Diabetes. 1990; 39: 1420-1424.
- Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem Journal. 1987; 245: 243-250.
- Chung SS, Ho EC, Lam KS, et al. Contribution of polyol pathway to diabetesinduced oxidative stress. Journal of the American Society of Nephrology. 2003; 14: 233-236.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414: 813-820.
- Wohaieb SA, Godin DV. Alterations in free radicals tissue-defence mechanisms in streptozotocin-induced diabetes in rat: Effect of insulin treatment. Diabetes. 1987; 36: 1014–1018.
- Srivastava P, Saxena AK, Kale RK, et al. Insulin like effects of lithium and vanadate on the altered antioxidant status of diabetic rats. Research communications in Chemical Pathology and Pharmacology. 1993; 80: 283–293.
- Kakar R, Kalra J, Mantha SV, et al. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Molecular and Cellular Biochemistry. 1995; 151: 113–119.
- Parinandi NL, Thomson EW, Schmid HHO. Diabetic heart and kidney exhibit increased resistance to lipid peroxidation. Biochem Biophys Acta. 1990; 1047: 63–69.
- Gupta BL, Baquer NZ. Hexokinase, glucose-6-phosphate dehydrogenase and antioxidant enzymes in diabetic reticulocytes. Effects of insulin and vanadate. Biochemistry and Molecular Biology International. 1998; 46: 1145–1152.
- Lyons TJ. Glycation and oxidation: A role in the pathogenesis of atherosclerosis. American Journal of Cardiology. 1993; 71: 26B–31B.
- Khandelwal RL, Pugazhenthi S. In vivo effects on hepatic glycogen metabolizing and lipogenic enzymes in insulin dependent and insulin resistant diabetic animals. Molecular and Cellular Biochemistry. 1995; 153: 7–94.
- Gupta D, Raju J, Jayaprakash R, et al. Changes in the lipid profile, lipogenic and related enzymes in the livers of experimental diabetic rats: Effect of insulin and vanadate. Diabetes Research and Clinical Practice. 1999; 46: 1–7.
- Maritim AC, Sanders RA, Watkins JB. Diabetes, Oxidative Stress, and Antioxidants: A Review. Biochem Molecular Toxicology. 2003; 17:1.
- Halliwell B, Gutteridge JMC. Free radical in biology and medicine (3rd edn). Oxford University Press, London. 1998; Chapter 3.
- Willcox O, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Critical Reviews in Food Science and Nutrition. 2004; 44: 275-295.
- Bajpai M, Pande A, Tewari SK . Phenolic contents and antioxidant activity of some food and medicinal plants. International Journal of Food Sciences and Nutrition. 2005; 56: 287-291.
- Ito N, Kukushima S, Hasegawa A, et al. Carcinogenecity of butylated hydroxyanisole in F344 rats. Journal of the National Cancer Institute. 1983; 70: 343-347.
- Abdollahi M, Larijani B, Rahimi R, et al. Role of oxidative stress in osteoporosis. Therapy. 2005; 2: 787-796.
- Fraga CG, ed. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. Hoboken, New Jersey: John Wiley & Sons, Inc; 2010.
- Rates SMK. Plants as source of drugs. Toxicon. 2001; 39: 603-613.
- Mukherjee PK, Kumar V, Mal M, et al. Acorus calamus: Scientific validation of ayurvedic tradition from natural resources. Pharmaceutical Biology. 2001; 45: 651-666.
- Syiem D, Syngai G, Khup PZ, et al. Hypoglycemic effects of Potentilla fulgens in normal and alloxan-induced diabetic mice. Journal of Ethnopharmacology. 2002; 83: 55-61.
- Syiem D, Majaw S. Effect of Potentilla fulgens L. aldose reductase activity of normal and diabetic mice. Inventi Rapid: Ethnopharmacology 2010; 1(1): ep13.
- Syiem D, Sharma R, Saio V. In vitro study of the antioxidant potential of some traditionally used medicinal plants of North-East India and assessment of their total phenolic content. Pharmacologyonline. 2009; 3: 952-965.
- Jaitak V, Sharma K, Kalia K, et al. Antioxidant activity of Potentilla fulgens: An alpine plant of western Himalayas. Journal of Food Composition and Analysis. 2010; 23: 142–147.
- Tomczyk M, Latte KP. Potentilla-A review of its phytochemical and pharmacological profile. Journal of Ethnopharmacology. 2009; 122: 184-204.
- Harborne JB, ed. Phytochemical Methods. New York: Chapman and Hall; 1984.
- Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979; 95: 351-358.
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Analytical Biochemistry. 1976; 72: 248-54.
- Aebi H. Methods in Enzymology. In: Catalse in vitro. Academy Press, New York. 1984; 105:121-126.
- Marklund SL, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974; 47:469-474.
- Rotruck JT, Pope AL, Ganther HE, et al. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973; 179: 588-590.
- Grankvist K, Marklund SL, Sehlin J, et al. Superoxide dismutase, catalase and scavengers of hydroxyl radical protect against the toxic actions of alloxan pancreatic islet cells in vitro. Biochemical Journal. 1978; 182:17–25.
- Abdollahi M, Ranjbar A, Shadnis S, et al. Pesticides and oxidative stress: A review. Medical Science Monitor. 2004; 10 (6): RA 144-RA 147.
- Smith CM, Marks AD, Lieberman MA. Oxygen and free radical injury: Marks Basic Medical Biochemistry: A clinical approach (2nd edn). Lippincott, Williams& Wilkins press; 2005.
- Basu S, Wiklund L, eds. Oxidative stress in applied basic research and clinical practice: Studies on experimental models. Humana Press; 2011.
- Aldini G, Yeum KJ, Niki E, Russell RM, eds. Biomarkers for Antioxidant Defense and Oxidative Damage: Principles and Practical Applications. Iowa, USA: Wiley and Blackwell; 2010.
- Sharma N, Garg V. Antidiabetic and antioxidant potential of ethanolic extract of Butes monosperma leaves in alloxan-induced diabetic mice. Indian Journal of Biochemistry and Biophysics. 2009; 46: 99-105.
- Anthony MU, Adebimpi AO, Ekpo KE. Antioxidant Potential of the young leave methanolic extract of Magnifera Indica in alloxan induced diabetic rat. Pakistan Journal of Nutrition. 2009; 8 (6): 716-720.
- Kennedy L, Baynes JW. Non-enzymatic glycosylation and the chronic complications of diabetes: An overview. Diabetologia. 1984; 26: 93-98.
- Van Dam PS, Van Asbeck BS, Erkelens W, et al. The role of oxidation stress in neuropathy and other diabetic complications. Diabetes Metabolism Review. 1995; 11: 181-192.
- Genet S, Kale RK, Baquer NZ Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum graecum). Molecular and Cellular Biochemistry. 2002; 236: 7-12.
- Veglioglu YS, Mazza G, Gao L. antioxidant activity and total phenolics in selected fruits, vegetables and grain products. Journal of Agriculture and Food Chemistry. 1998; 46: 4113-4117.