Development and validation of reversed-phase high-performance liquid chromatography method for estimation of rizatriptan benzoate in oral strip formulations
- *Corresponding Author:
- Dr. Krishnamurthy Bhat
Department of Pharmaceutical Quality Assurance, Manipal College of Pharmaceutical Sciences, Manipal University, Manipal - 5761 04, Karnataka, India.
E-mail: km.bhat@manipal.edu
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Aim: A simple, accurate, precise, and reproducible reversed‑phase high‑performance liquid chromatography (RP‑HPLC) method was developed and validated for the determination of rizatriptan benzoate in oral strip formulations. Methodology: Separation was achieved under optimized chromatographic condition on a Hiper C18 column (250 mm × 4.6 mm, 5 m) using Shimadzu HPLC. The mobile phase consisted of phosphate buffer (20 mM pH adjusted to 3.2 ± 0.005 with ortho phosphoric acid): Methanol in the ratio of 70:30 v/v with isocratic elution at a flow rate of 1 ml/min at ambient temperature was performed. The detection was carried out at 225 nm using photodiode array detector. The method was validated as per Q1A (R2) guidelines and suitability of developed method was ascertained by using optimized oral strip formulation. Results: The retention time of rizatriptan benzoate was found to be 5.17 min, and the calibration curve was linear in the concentration range of 0.20–20 mg/mL (r2 = 0.9998). The limit of detection and the limit of quantitation were found to be 0.016 mg/mL and 0.0528 mg/mL, respectively. Method validation parameters were found to be within the specified limits. The percentage drug content of oral strips formulation was found to be 98.96 ± 1.37. Conclusion: The proposed HPLC method may be used efficiently for routine and quality control analysis of rizatriptan benzoate in pharmaceutical formulations.
Keywords
Liquid chromatography, oral strips, rizatriptan benzoate, validation
Introduction
Migraine is a neurological syndrome characterized by altered body perceptions, severe headaches, and nausea. Physiologically, the migraine is a neurological condition, which is about three times more common to women than men.[1] Rizatriptan benzoate is known chemically as N, N-dimethyl-5-(1H-1,2,4-triazol-1- ylmethyl)-1H-indole-3-ethanamine monobenzoate [Figure 1], binds to human 5-hydroxytryptamine 1B/1D receptor with high affinity and selectivity. Several clinical trials reveal the effectiveness of oral rizatriptan for the treatment of a migraine headache.[2-4]
After oral administration, a peak plasma rizatriptan concentration was obtained in about 1–1.5 h depending on the formulation and bioavailability was found to be 40–45%. Food may delay the peak plasma concentrations of the tablet formulation by about 1 h. Whereas, only 14% of drug will bind to plasma proteins. Rizatriptan metabolized primarily by MAO type A to inactive indole acetic acid derivative (14%) and to active N-monodesmethyl rizatriptan (1%).
Oral strips have several unique and novel advantages that make them better than orally disintegrating dosage forms. The fast dissolving thin strip is simply placed on the patient tongue or any oral mucosal tissue, immediately wet by saliva resulted in rapid hydration and adheres on to the site of application. It then rapidly disintegrates and dissolves to release the medication for faster absorption. The oral strips are very thin, which ensure rapid disintegration due to the larger surface area available for wetting and eventual dissolution.[5,6] Further, the determination of rizatriptan in bulk drug or pharmaceutical dosage forms were reported using liquid chromatography with tandem mass spectrometry and fluorescence detection system.[7-9] The present study deals with a simple, sensitive and rapid development and validation of rizatriptan benzoate in oral strips using reversed-phase high-performance liquid chromatography (HPLC) with photodiode array detector.
Materials and Methods
Materials and reagents
Rizatriptan benzoate was gifted from Dr. Reddy’s laboratories Hyderabad, India. HPLC grade methanol was purchased from Finar Ltd., Ahmedabad and all other chemicals, polymers and plasticizers were purchased from Loba Chemie Pvt. Ltd. Mumbai.
Methodology
Preparation of oral strips
Oral strips were prepared by solvent casting technique.[9] In this method, water-soluble ingredients were dissolved to form a clear viscous solution. The active pharmaceutical ingredient and other agents were dissolved in smaller amounts of the solvent and combined with the bulk. The resulting solution was cast as a film and allowed to dry, which was then cut into pieces of the desired size.
Chromatographic system and conditions
A standard solution consisting of rizatriptan (5 μg/mL) was prepared in methanol and 20 mM phosphate buffer (pH 3.2) (30:70 v/v)] and chromatographed on the following conditions:
• Stationary phase: Hyper C-18, (25.0 cm × 4.6 mm, 5.0 μm)
• Mobile phase A: Methnol
• Mobile phase B: 20.0mM phosphate buffer (pH 3.2 ± 0.005)
• pH was adjusted to 3.2 with orthophosphoric acid
• Detection wavelength: 225 nm
• Run time: 8 min
• Column oven temperature: 25°C
• Flow rate: 1 mL/min
• Injection volume: 20.0 μL
• Temperature: Ambient
• Auto sampler temperature: 25°C.
Method validation
The method is validated as per ICH Q2 (R1) (Validation of Analytical Procedures: Text and Methodology) guideline. Typical validation characteristics such as accuracy, precision, repeatability, intermediate precision, specificity, detection limit (LOD), quantitation limit (LOQ), linearity, range and robustness were evaluated.[10-12]
Precision
Method precision (repeatability)
Method precision (repeatability) is the result of the method operating over a short time interval under the same conditions (inter-assay precision). Six replicates of standard solution comprising of rizatriptan benzoate (5.0 μg/mL), was prepared, and 20.0 μL was injected in duplicate and chromatograms were recorded. The mean area and % relative standard deviation (RSD) for those injections were calculated.
Intermediate precision (ruggedness)
The procedure followed for assay method in repeatability was carried out on two different days, by two different analyst and using two different HPLC systems. Six replicates of standard solution comprising of rizatriptan benzoate (5.0 μg/mL) was prepared and injected in duplicate and chromatograms were recorded. The mean area and % RSD for those injections were calculated.
Linearity
The linearity of an analytical method is its ability to obtain a test result, which has a definite mathematical relationship to the concentration of analytes. A standard stock solution of rizatriptan benzoate (100.0 μg/mL) was used to prepare the linearity solution of desired concentrations. Different volumes of stock solutions were accurately transferred into 10 ml volumetric flasks and diluted to mark to yield 0.2–20.0 μg/mL concentration range. Eight solutions of 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 15.0 and 20.0 μg/mL was prepared. For each of the above concentrations duplicate injections (20.0 μl) were performed (n = 4). From the peak area of duplicate injection of the drugs, mean area and % RSD was calculated. The calibration curve was constructed by using ratios of the observed analyte peak area versus concentration of analyte. The regression coefficient (r2), y-intercept, slope of the regression line were determined by linear regression data analysis.
Accuracy
The accuracy of the method was performed by recovery study. Known amount of standard drug was spiked to the preanalyzed samples and recovery of the drug was calculated. Accuracy was performed at three levels of 80%, 100% and 120% standard concentration. A solution containing 5.0 μg/mL of the sample was spiked with 80% 100% and120% of the standard solution of rizatriptan benzoate. Accuracy of the assay method was evaluated under selected chromatographic conditions. The % recovery was calculated at each level from the peak area of the drug.
Robustness
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage.
Change in pH of the diluent (phosphate buffer)
The pH of the buffer present in the mobile phase was changed by ±0.2 unit that is pH was adjusted to 3.0 and 3.4. Single injection of blank preparations (20.0 μL) and six replicates of standard solution of rizatriptan benzoate (5.0 μg/mL), were injected. Symmetry factor and relative retention time (RRT) was calculated.
Change in mobile phase composition
The organic phase composition was changed by ±2%. Single injection of each blank preparation (20.0 μL) and six replicates of standard solution were injected, for each change symmetry factor and RRT was calculated.
Change in flow rate
The flow rate was changed to 0.9mL/min and 1.1 mL/min (±0.1 ml/min). Each blank (20 μL; one injection) and standard solution (six injections) were injected for each of the above mentioned flow rate. Symmetry factor and RRT was calculated for the above mentioned condition.
Change in wavelength of detection
The wavelength was changed by ±5.0 nm (220 nm and 230 nm). Single injection of 20.0 μl of each blank and standard solution (six injections), were injected to check for the compliance of the system suitability test for two wavelengths and RRT was calculated.
Analysis of formulations
The optimized oral strip formulation was analyzed using a validated method. Twenty strips (2 cm × 2 cm) were weighed, and average weight was calculated. An amount equivalent to 50.0 mg of Rizatriptan benzoate was dissolved in 100 mL Milli Q water. The solution was sonicated for 10 min and filtered. The first portion of the filtrate was discarded. The obtained clear solution was used as stock sample solution. The stock solution was further diluted quantitatively with diluent (methanol: Phosphate buffer [pH 3.2] 30:70 v/v) to obtain the working concentration range of 0.2–20.0 μg/mL rizatriptan benzoate in a 10 ml volumetric flasks and volume was made up with diluent.
The sample was subjected to HPLC analysis. 20.0 μL of blank (one injection) and sample solution (six replicate injections) were injected, and % recovery of the analytes was calculated.
Results and Discussion
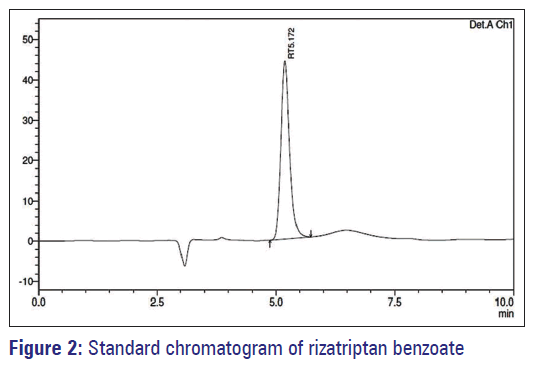
The method was validated as per ICH Q2 (R1) guideline. The method was validated as per ICH Q2 (R1) guideline. The retention times of Rizatriptan benzoate was 5.17 min. The typical chromatogram was given in Figure 2.
Method validation by reversed-phase high-performance liquid chromatography
System suitability
The overall system suitability (n = 6) was evaluated for the system suitability of the proposed method. The high counts of theoretical plates revealed the column efficiency. The % RSD showed that the proposed method is precise. The summary of system suitability parameter was expressed in Table 1.
| System suitability parameter* | Observation | Acceptance criteria |
|---|---|---|
| Tailing factor | 1.17±0.07 | Should NMT 2.0 |
| Theoretical plates/meter (n) | 8962±38 | Should NLT 2000 |
*Average of 6 determinations (n=6). HPLC: High-performance liquid chromatography, NMT: Not more than, NLT: Not less than
Table 1: Summary of system suitability studies of assay method by HPLC
Specificity
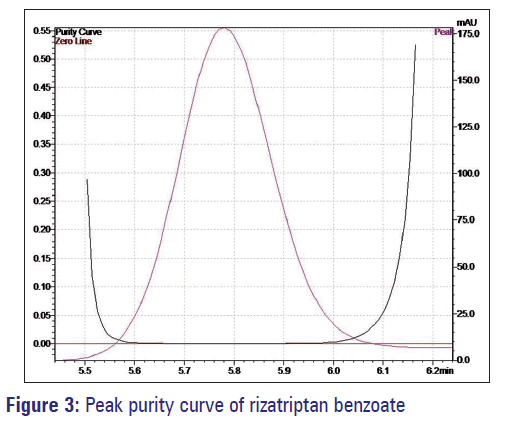
The developed method was used for the estimation of rizatriptan benzoate in oral strips. The diluent did not interfere in the estimation when it was evaluated for the spectral purity in the diode array detector, the purity of the peak constituting for rizatriptan benzoate pass the test. The total peak purity index (0.9999) was found to be greater than a single point threshold (0.9809). It indicates that the method is highly specific, and no other components were co-eluting with main analyte [Figure 3].
Precision
Method precision (repeatability)
To perform this parameter of validation study six replicates (20.0 μL) of standard preparation consisting of rizatriptan benzoate (5.0 μg/mL), was injected and % RSD of concentration was calculated and presented in Table 2.
| Set number | Method precision (5 µg/ml) |
|---|---|
| 1 | 5.01 |
| 2 | 5.001 |
| 3 | 5.01 |
| 4 | 5.00 |
| 5 | 5.00 |
| 6 | 5.01 |
| Mean | 5.00 |
| SD | 0.003 |
| % RSD | 0.07 |
SD: Standard deviation, RSD: Relative standard deviation
Table 2: Results of the method precision
Intermediate precision (ruggedness)
The procedure followed for assay method in method precision was repeated on two different days and given Table 3.
| Concentration (µg/ml) | Measured concentration±SD; intra-day (n=6) |
% RSD | Measured concentration±SD; inter-day (n=6) |
% RSD |
|---|---|---|---|---|
| 5 | 5.00±0.003 | 0.07 | 5.07±0.005 | 0.1 |
SD: Standard deviation, RSD: Relative standard deviation
Table 3: Precision data evaluated through intra-day and inter-day studies
Linearity and range
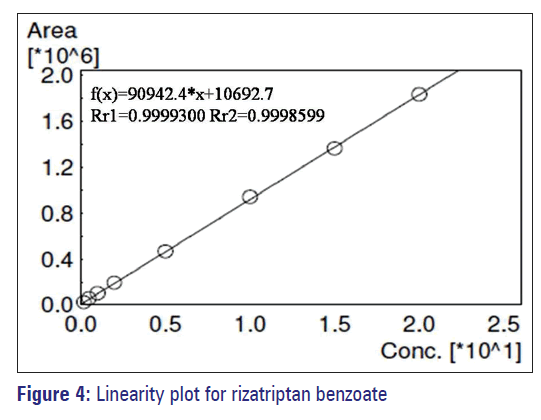
The calibration curves (n = 4) were constructed over the concentration range between 0.20 and 20mg/ml. The regression coefficient was 0.9998, and the results indicate that the method was linear, precise and accurate and given in Table 4 and Figure 4.
| Concentration (µg/ml) | Area response |
|---|---|
| 0.200 | 24,380 |
| 0.500 | 55,115 |
| 1.000 | 100,987 |
| 2.000 | 193,587 |
| 5.000 | 464,916 |
| 10.000 | 937,191 |
| 15.000 | 1,362,888 |
| 20.000 | 1,830,083 |
| Regression coefficient (r2) | 0.9998 |
Table 4: The linearity level
Accuracy
The accuracy of the method was performed by recovery studies. The known amount of standard drug (80%, 100% and 120% w/v) was spiked in triplicate to the preanalyzed sample solution, and the recovery of the drug was calculated. The percentage recoveries at three different concentrations (4, 5 and 6 μg/mL) were found to be 101.31 ± 0.074, 102.27 ± 0.124 and 101.02 ± 0.196 for rizatriptan benzoate and given in Table 5.
| Level % | Added amount (μg/ml) |
Amount recovered (µg/ml) |
% recovery |
Mean % recovery±SD |
|---|---|---|---|---|
| 80 | 4 | 4.05 | 101.35 | 101.31±0.074 |
| 4 | 4.05 | 101.35 | ||
| 4 | 4.04 | 101.22 | ||
| 100 | 5 | 5.11 | 102.25 | 102.27±0.124 |
| 5 | 5.1 | 102.17 | ||
| 5 | 5.12 | 102.41 | ||
| 120 | 6 | 6.06 | 100.96 | 101.02±0.196 |
| 6 | 6.05 | 100.86 | ||
| 6 | 6.07 | 101.24 | ||
| Across all levels | 101.53±0.66 |
SD: Standard deviation,
Table 5: Accuracy of the assay method
Limit of detection and limit of quantitation
The LOD and LOQ of the developed method were calculated based on the standard deviation of the response and the slope. The calculated LOD and LOQ values of the Rizatriptan benzoate were found to be 0.016 mg/mL and 0.0528 mg/mL respectively.
Robustness
The robustness of the method was evaluated by varying method parameter such as pH, wavelength, % organic content, and determining the affect (if any) on the results of the method.
Change in pH of the buffer
The pH of the buffer (phosphate buffer pH 3.2) was a change by ±0.2 unit that is buffer pH 3.0 and 3.4. The relative retention time of the peak was given in Table 6.
| pH | 3.2 | 3.0 | 2.8 |
|---|---|---|---|
| Men pek re | 463,325 | 501,231 | 452,789 |
| Rt (min) | 5.16 | 5.02 | 4.96 |
| RRT | 1.01 | 0.92 | 0.89 |
| Tiling fctor | 1.12 | 1.27 | 1.03 |
RRT: Relative retention time
Table 6: Effect of change in the buffer pH of mobile phase on chromatographic profile
Change in flow rate
The flow rate change by ± 0.1 mL/min, and its effect on RRT and asymmetry was observed and given in Table 7.
| Flow rate | 1.0 | 0.9 | 1.1 |
|---|---|---|---|
| Mean peak area | 464,928 | 485,342 | 494,235 |
| Rt (min) | 5.12 | 4.97 | 4.84 |
| RRT | 1.0 | 1.21 | 0.92 |
| Tailing factor | 1.21 | 1.01 | 1.03 |
RRT: Relative retention time
Table 7: Effect of change in the flow rate of mobile phase on chromatographic profile
Change in mobile phase composition
The organic phase composition was changed by ±2% and its effect on RRT and asymmetry was observed and given in Table 8.
| Mobile phase composition | 70:30 | 68:32 | 72:32 |
|---|---|---|---|
| Mean peak area | 464,356 | 487,456 | 462,568 |
| Rt (min) | 5.17 | 5.01 | 5.14 |
| RRT | 0.53 | 0.65 | 0.75 |
| Tailing factor | 1.03 | 0.97 | 1.18 |
RRT: Relative retention time
Table 8: Effect of change in the mobile phase composition on chromatographic profile
Change in wavelength
The wavelength was changed by ± 5 nm and its effect on RRT was observed. The RRT was given in Table 9.
| λ (nm) | 225 | 220 | 230 |
|---|---|---|---|
| Mean peak area | 483,456 | 456,904 | 501,267 |
| Rt (min) | 5.17 | 5.10 | 4.92 |
| RRT | 0.92 | 0.67 | 0.95 |
| Tailing factor | 1.12 | 1.10 | 0.96 |
RRT: Relative retention time
Table 9: Effect of change in detection wavelength on chromatographic profile
Analysis of formulations
The developed HPLC method was applied to the determination of rizatriptan benzoate in Optimized Oral strip preparation.
Assay for the Oral strips formulation was found to be 98.96 ± 1.37.
Conclusion
All validation parameters for proposed HPLC instrument and method were within the acceptance range. And they were found to be specific, linear, precise, accurate and economic. The proposed procedure could be applied efficiently for routine and quality control analysis of rizatriptan benzoate in pharmaceutical formulations with higher precision and accuracy.
References
- Stovner LJ, Zwart JA, Hagen K, Terwindt GM, Pascual J. Epidemiology of headache in Europe. Eur J Neurol 2006;13:333-45.
- Brunton L, Parler K, Blumenthal D. Goodman and Gillman’s Manual of Pharmacology and Therapeutics. USA: McGraw-Hill Medical Publishing Division; 2008.
- Barbanti P, Fofi L, Dall’Armi V, Aurilia C, Egeo G, Vanacore N, et al. Rizatriptan in migraineurs with unilateral cranial aut onomic symptoms: A double-blind trial. J Headache Pain 2012;13:407-14.
- Seeburger JL, Taylor FR, Friedman D, Newman L, Ge Y, Zhang Y, et al. Efficacy and tolerability of rizatriptan for the treatment of acute migraine in sumatriptan non-responders. Cephalalgia 2011;31:786-96.
- Dixit RP, Puthli SP. Oral strip technology: Overview and future potential. J Control Release 2009;139:94-107.
- Mishra R, Amin A. Formulation and development of taste-masked rapidly dissolving films of Cetrizine hydrochloride. Pharm Tech 2009;33:48-56.
- Guo JF, Zhang AJ, Zhao L, Sun XH, Zhao YM, Gao HZ, et al. Determination of rizatriptan in human plasma by liquid chromatographic-eletrospray tandem mass spectrometry: Application to a pharmacokinetic study. Biomed Chromatogr 2006;20:61-6.
- Chen Y, Miao H, Lin M, Fan G, Hong Z, Wu H, et al. Development and validation of a selective and robust LC-MS/MS method for high-throughput quantifying rizatriptan in small plasma samples: Application to a clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2006;844:268-77.
- Chen J, Jiang X, Jiang W, Mei N, Gao X, Zhang Q. Liquid chromatographic method for the determination of rizatriptan in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2004;805:169-73.
- Indira Muzib Y, Reddy J, Chowdary KP, Swathi E. Development and validation of RP-HPLC method for estimation of tapentadol hydrochloride in bulk and tablet dosage forms. Int J Chem Anal Sci 2013;4:67-72.
- Arayne MS, Sultana N, Zuberi MH. Development and validation of RP-HPLC method for the analysis of metformin. Pak J Pharm Sci 2006;19:231-5.
- Mallikarjuna Rao B, Sangaraju S, Srinivasu MK, Madhavan P, Lalitha Devi M, Rajendra Kumar P, et al. Development and validation of a specific stability indicating high performance liquid chromatographic method for rizatriptan benzoate. J Pharm Biomed Anal 2006;41:1146-51.