Design and evaluation of hydrophobic coated buoyant core as floating drug delivery system for sustained release of cisapride
- *Corresponding Author:
- Shery Jacob
Faculty of Pharmacy, Misruta University, Al-Khoms, Libya
E-mail: sheryjacob@ymail.com
Date of Received :22-11-2011
Date of Modified :25-12-2011
Date of Accepted :08-01-2011
Available Online :15-02-2011
Abstract
An inert hydrophobic buoyant coated–core was developed as floating drug delivery system (FDDS) for sustained release of cisapride using direct compres-sion technology. Core contained low density, porous ethyl cellulose, which was coat-ed with an impermeable, insoluble hydrophobic coating polymer such as rosin. It was further seal coated with low viscosity hydroxypropyl methyl cellulose (HPMC E15) to minimize moisture permeation and better adhesion with an outer drug layer. It was found that stable buoyant core was sufficient to float the tablet more than 8 h with-out the aid of sodium bicarbonate and citric acid. Sustained release of cisapride was achieved with HPMC K4M in the outer drug layer. The floating lag time required for these novel FDDS was found to be zero, however it is likely that the porosity or density of the core is critical for floatability of these tablets. The in vitro release pattern of these tablets in simulated gastric fluid showed the constant and controlled release for pro-longed time. It can be concluded that the hydrophobic coated buoyant core could be used as FDDS for gastroretentive delivery system of cisapride or other suitable drugs.
KeyWords
Floating drug delivery system, hydrophobic buoyant core, coating, cisapiride.
Introduction
The key challenge in the oral delivery of drugs acting locally in the stomach or having narrow absorption window in the gastrointestinal tract is the retention of the dosage form in the upper gastrointestinal tract for lingering therapeutic effect. Several approaches have been attempted to prolong the gastric retention of solid dosage forms in the stomach including mucoadhesion, floatation, sedimentation, expansion, modifying shape, or by simultaneous administration of pharmacological agents that delay gastric emptying. Among these, FDDS or hydrodynamic balanced systems (HBS) have demonstrated to be the promising method due to its capacity to retain the dosage form in the stomach for a predetermined time [1] .Several parameters such polymers, dosage form density, formulation and processing variables, addition of gas forming agents etc affects the successful delivery of a floating drug delivery systems, and needs prime consideration. The choice of the polymer is based on its bulk density, ability to form a cohesive gel barrier, and capability to control the release of the drug over a period of time [2, 3].
Hydrophilic polymers are widely investigated and found to be the most apposite in controlling the release of drug in floating drug delivery systems. Matrix tablets based on HPMC, upon contact with gastric fluid, the systems takes up water and swell. As the increase in volume is greater than increase in mass during swelling, the densities of these devices decrease and the system start to float after a short lag time [4]. Conventional method using HPMC as polymer in FDDS was published in recent literature [5, 6]. Alternatively, the induction of floatation could be modified by the incorporation of sodium bicarbonate and citric acid, gas forming agents dispersed in HPMC hydrogel matrix [7, 8]. Further, reduced floating lag time could be achieved by reducing the compression force, increasing polymer molecular weights and increasing the particle sizes of the matrix forming polymer [9]. Modification of the dosage form has been also attempted by formulating floating device consisting of two drug coated HPMC matrix tablets within an impermeable hollow polypropylene cylinder open at both ends [10]. Several approaches have been made to develop low density solid systems using sponges or highly porous structures such as foam or a hollow body to prolong the floating of dosage forms [11]. Recently calcium silicate based porous carrier based floating orlistat microspheres for gastric emptying was carried out by Jain et al [12].
In the current investigation it is hypothesized that the incorporation an auxiliary inert low density porous core into the dosage form could be useful for the rapid floating which can persist for a prolonged period of time. The floating drug delivery system designed consists of an inert core tablet (porous ethyl cellulose) of low density, encompassing the drug containing HPMC matrix. The core was seal coated with an impermeable water insoluble polymer (rosin), which retain its porosity, and was film coated with low viscosity HPMC E15, provided a barrier to moisture permeation and better adhesion of core and outer drug coat. The proposed dosage form was formulated and evaluated In vitro using a model drug, cisapiride, possessing strong pH dependent solubility.
Materials And Methods
Materials
Cisapride (Dr. Reddy's Laboratories Ltd, Hyderabad, India), methocel E15, and methocel K4M (Colorcon Asia Pvt Ltd., Goa, India) were obtained as gratis sample. Ethyl cellulose (ethocel Std premium 4, Dow Chemical Company, USA), carbopol (carbomer 971) (BF Goodrich Chemical, USA), rosin N Grade (Swastik Acids and Chemicals, Nagpur, India), magnesium sterarate, colloidal silicon dioxide, sodium bicarbonate, PEG 400, citric acid anhydrous (SD FineChem Ltd, Ahmedabad, India) were purchased commercially. All other ingredients were of reagent grade. Double distilled water was used throughout the study.
Preparation of Core-Coat Floating Tablets
Core tablets
Ethyl cellulose (100 mg) was taken as core, was previously passed through 590 μm sieve (mesh # 30) to obtain uniform particle size. It was directly compressed by 8 x 2.5 mm deep concave punches using electrically operated single punch tablet machine (Cadmach, Mumbai, India).The average core tablet hardness was 30–50 N.
Sub and seal coating
The prepared core tablets were sub coated at 15% w/w level using water insoluble polymer, rosin by dip coating method. The solvent and plasticizer (0.1%w/w) used were acetone and PEG 400, respectively. It was further seal coated at 3% w/w using HPMC E15 in aqueous medium.
Outer drug layer
The outer drug layer consists of either alone or combination of HPMC K4M and sodium bicarbonate, citric acid with cisapride. It was previously passed through 210 μm sieve to obtain uniform dispersed mixture. Half of the mixture was filled into die cavity and the seal coated tablet was placed over it. Remaining half of the mixture was added and then compressed using 12 x 3.5 mm deep concave punches using electrically operated single punch tablet machine. The prepared formulation with core-coat was denoted as F2.The average final tablet hardness was 30–50 N.
Preparation of floating tablets
Formulations with no central core (F1, F3, and F4 ) have been prepared by mixing the respective ingredients and compressed directly using electrically operated single punch tablet machine (Cadmach, Mumbai, India).The average tablet hardness was 30–50 N.
Evaluation Of Tables
Determination of tablet density
The apparent densities of the tablet samples were determined based on their volumes and masses. The height and diameter of the prepared tablets measured using micrometer gauges were used for the calculation of the volume of the approximate cylindrical devices. The true density of the tablets were measured using a helium pycnometer (Accupyc 1330, Micromertics, USA) and done in triplicate. The porosity ε, of the samples was calculated using the equation: ε = 1 – ρa/ρt, where ρa represents the apparent density and ρt the true density of the samples.
Determination of floating behaviour
This magnitude of floating strength may vary as function of time and usually decreases after immersion of dosage form into the fluid as a result of the development of its hydrodynamic equilibrium [13]. To monitor the total vertical force F acting on an immersed object, a modified apparatus according to Sandra et.al was used [14]. The resultant weight of the bouyant tablet can be described by the following equation,
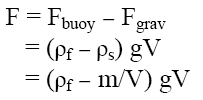
where F is the total vertical force, g the acceleration due to gravity, ρf the density of the fluid, ρs the density of the tablet, m the tablet mass and V the tablet volume respectively.
The instrument used to determine the floating strength of the tablet samples actually measured the force 'F' required to maintain the tablet totally submerged into the dissolution medium. A sample holder was connected to a metal base placed on an analytical balance via a metal pole. For the performance of floating strength experiments the tablets were placed in beaker with 900 ml of 0.1N HCl at 37°C, so that the sample holding device was covered with dissolution medium. The floating strength was then determined as the weight reduction on the analytical balance over certain period of time. Floating lag time, and the floating duration of different formulations were measured. All experiments were performed in triplicate.
In vitro dissolution tests
The cisapride release from different floating formulation was determined using a USP 23 paddle apparatus 2 under sink conditions. The dissolution medium was 900 ml simulated gastric fluid (pH 1.2, no enzyme) at 37± 0.2°C; paddle speed 50 rpm, to simulate in vivo conditions. All experiments were done in triplicate and average values were taken. The prepared formulation was subjected to dissolution tests for 8 h. Samples (2 ml) were withdrawn at predetermined time intervals, filtered through millipore membrane filter and was replaced by an equal volume of dissolution medium. Drug content in the sample was analysed by HPLC method using mobile phase of acetonitrile in 0.02 M phosphate buffer (pH 5.2) at 1.0 ml/min through a C8 symmetrical column. Cisapride and the internal standard were detected by fluorescence monitoring at 295 nm (excitation) and 350 nm (emission) [15].
Kinetics of drug release
The In vitro release data of the prepared formulations were evaluated kinetically by zero order kinetics, first order kinetics, Higuchi and ideal kinetic models [16].
Statistical Analysis
The data were tested by one-way analysis of variance (ANOVA) and t-test using graphpad prism 5 (graphpad software, Inc., CA, USA) to test the effects of various treatments. P value less than 0.05 was considered statistically significant. The data points provided in the graph is an average of six trials. The error bars represents the standard deviation.
Results And Discussion
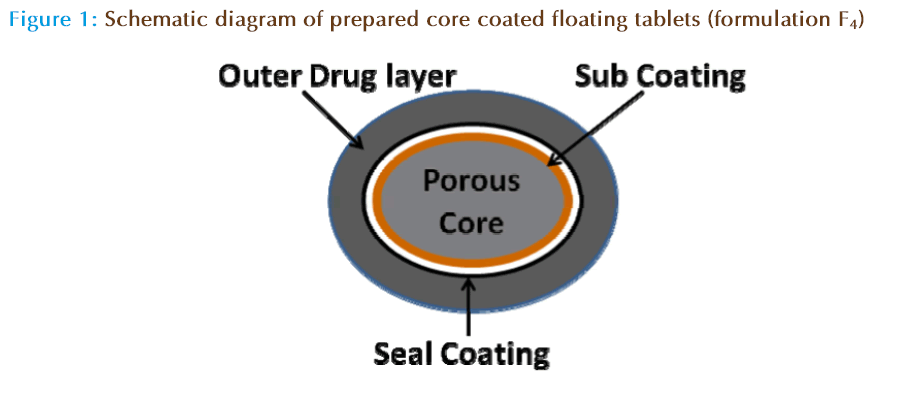
Four formulations were prepared (F1 – F4) and the detailed composition is given in Table 1. Formulations (F1, F3, and F4) were made by mixing all the ingredients and compressed in a single punch tablet press. However, formulation F2 has been prepared in two steps, initially a core part was prepared using ethyl cellulose and was coated. Secondly, the coated core tablets were encircled with the drug containing HPMC matrix, from where the release of drug is controlled. The formulation F1 (conventional floating tablet) was fabricated by incorporating gas generating agents such as sodium bicarbonate (60 mg/ tablet) and citric acid (20 mg/tablet), however, in formulation F3 citric acid was not employed. This is because in hydrogen ion concentration in acidic environment of stomach will initiates the formation of carbon dioxide. In contrast, formulations F2 and F4 were devoid of any gas generating agents and have similar composition. Fundamental differences between these two formulations (F2 and F4) are regarding the added separate ethyl cellulose core, which was not included in formulation F4. The quantity of ethyl cellulose was kept constant (100 mg) in all the formulations for comparison studies. The structure of the proposed core based floating tablet (F2 ) is shown schematically in Figure 1.
| Ingredients (mg) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Ethyl cellulose | 100 | 100 | 100 | 100 |
| HPMC K4M | 500 | 500 | 500 | 500 |
| Sodium bicarbonate | 60 | 60 | ||
| Citric acid | 20 | - | - | - |
| Magnesium stearate | 1.0 | 1.0 | 1.0 | 1.0 |
| Aerosil | 4.5 | 4.5 | 4.5 | 4.5 |
| Cisapiride | 5 | 5 | 5 | 5 |
Table 1: Composition of the prepared formulation
For formulation F2 , core was made of porous, low density (0.4 g/cc) ethyl cellulose which has very less density when compared to the gastric fluid (1.0 g/ cm3). Large particle size of ethyl cellulose was used to create maximum air entrapment inside the core material. Further, the punching of core was done at low compression force (10 MPa) to retain porosity, and could eventually leads to a low density system. Moreover, the compression was carried out using deep concave punches where the compression force will be distributed over the edges rather than concentrate at the core and thereby prevent the damage of the central core.
In the next stage, core was subcoated (up to 15% w/w) with water insoluble and impermeable natural coating polymer, rosin, which is a biodegradable thermoplastic solid resin obtained naturally from the trees of Pinus species [17, 18]. Review of the literature indicates that rosin and its derivatives are widely used in pharmaceutical as a coating agent, paint and paper industries for several applications [19–21]. Low molecular weight polyethylene glycol (MW 400) was used as plasticizer to improve the mechanical properties and to provide suitable flexibility to the coating polymer. The sub coated core was further film coated with low viscosity HPMC (E15) to provide moisture protection and better adhesion between core and drug loading section. In addition to colloidal silicon dioxide, HPMC acts as cushioning agent which will protect the polymer fracture during compression process [16].
The apparent tablet densities of all samples were found to be lower than 1.004 g/cm3 (Table 2). Among the formulations, the lowest density was observed with the formulation F2 , likely due to the stable air entrapped core. Similarly, the percentage porosity was also found to be higher in formulation F2 , compared to formulations without core (F1, F3 and F4). It is worthy to mention here that in case of formulations F1 , F3 and F4, the density will further increase and the porosity will decrease in presence of the medium periodically. The reason for the same could be the gradual loss of air bubbles by the diffusion of water over certain period of time.
| Formulations | Apparent density (g/cc) | True density (g/cc) | Percentage porosity |
|---|---|---|---|
| F1 | 0.91± 0.07 | 1.145 ± 0.11 | 25.5 ± 0.26 |
| F2 | 0.69 ± 0.11 | 0.90 ± 0.06 | 35.6 ± 0.87 |
| F3 | 0.92 ± 0.23 | 1.140 ± 0.26 | 24.9 ± 0.45 |
| F4 | 0.97 ± 0.09 | 1.152 ± 0.18 | 21.8 ± 0.98 |
* Each value is the mean ± SE (n= 6)
Table 2: Apparent density, true density and porosity of various cisapiride floating tablets*
The floating behaviour (onset time and duration of floating) of the prepared formulations were assessed and depicted in Table 2. As expected tablets with HPMC K4M, sodium carbonate and/or citric acid (F1 and F3) sank before floating, showing floating lag times of between 2 to 3 min. The lag time signifies the time taken by the formulation to produce sufficient amount of carbon dioxide bubbles, which facilitate the floating of tablets. Among the formulations (F1 and F3), the lag time of F1 was found to be lesser than F3 due to the presence of citric acid, which will produce an immediate acidic environment inside the matrix for the rapid formation of carbon dioxide bubbles. In contrast, the core based formulation (F2) floated immediately upon contact with the liquid medium, showing no lag times in floating behaviour probably because of the very low density of the formulation (t = 0, Table 3).The floating duration for formulation F2 was (~24 h), although the entire drug release has completed in 8 h. This indicates that the drug release could be extended upto 24 h by suitable substitution with high viscosity HPMC. However, the conventional formulation F4 could not float as it was without any floating mechanism.
| Formulations | Floating onset time (min)a | Floating duration (h)b | Zero order kinetics | Higuchi model |
|---|---|---|---|---|
| F1 | 1 – 3 ± 0.28 | 8 – 9 ± 0.45 | 0.9976 | 0.9778 |
| F2 | 0 | >24 | 0.9888 | 0.9886 |
| F3 | 3 – 4 ± 0.18 | 8 – 9 ± 0.65 | 0.9786 | 0.9856 |
| F4 | –c | – | 0.9443 | 0.9678 |
aThe fl oating onset time (time period between placing FDDS in the medium and beginning of bouyancy).
bFloating duration of FDDS
CNot occured
*Each value is the mean ± SE (n= 6)
Table 3: Floating ability and release kinetics of various formulation of floating tablets*
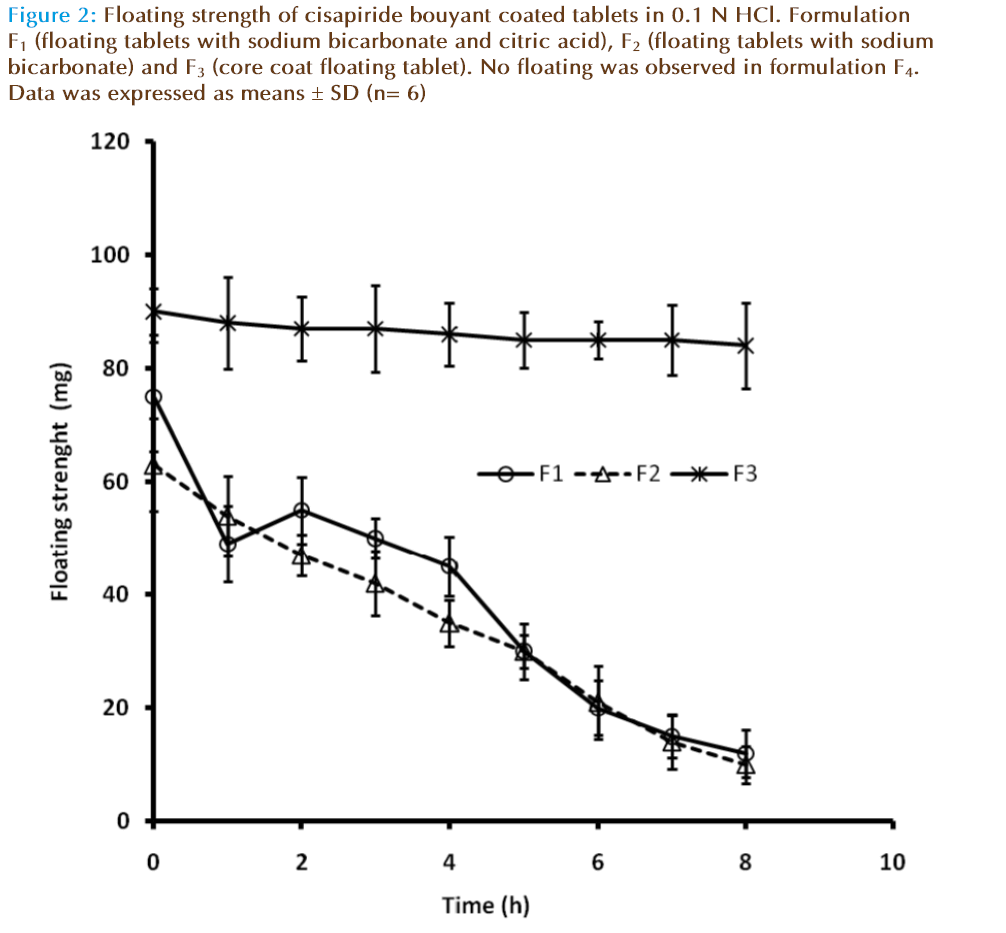
As shown in Figure 2 the floating strength was directly related to gas entrapment with respect to time. The floating strength of F2 remains constant for longer period of time because of the stable porous core. The floating strength of F1 is little more than F3 may due to the immediate formation of carbon dioxide bubbles because of the presence of both citric acid and sodium bicarbonate. This was observed by the increase in volume of the tablet without signifi - cant swelling and therefore initially slight reduced floating strength values for F1 .The water diffusion in the matrix is capable of solvation of the polymer and slowly collapsing the entrapped air from the matrix. This results in the gradual loss of floating strength of F1 and F3. Floating of core continued over 24 h for F2 with terminal weight reduction about 25 mg while tablets F1 and F3 disintegrated after 9 h. In in vivo the tablet has to float in presence of food, which might greatly increase the viscosity. Higher floating forces increase the probability of the tablets to remain afloat, this attributes plays a major role to reduce food effects on tablet retention.
Figure 2: Floating strength of cisapiride bouyant coated tablets in 0.1 N HCl. Formulation F1 (floating tablets with sodium bicarbonate and citric acid), F2 (floating tablets with sodium bicarbonate) and F3 (core coat floating tablet). No floating was observed in formulation F4. Data was expressed as means ± SD (n= 6)
It was also found that at high compression, the lag time was increased due to the delay in the formation of carbon dioxide bubbles (data not shown). The stable buoyant core (in F2 ) will support the external drug layer even after the loss of air bubbles in the slowly dispersible or soluble hydrogel like HPMC as common in FDDS. So this major disadvantages of typical FDDS can be eliminated by the novel stable core based FDDS. We have verified this by substituting low density ethyl cellulose with high density material like carbopol (2.08g/cc) [19]. It is critical that the tablets must be compressed at low compression force to retain porosity.
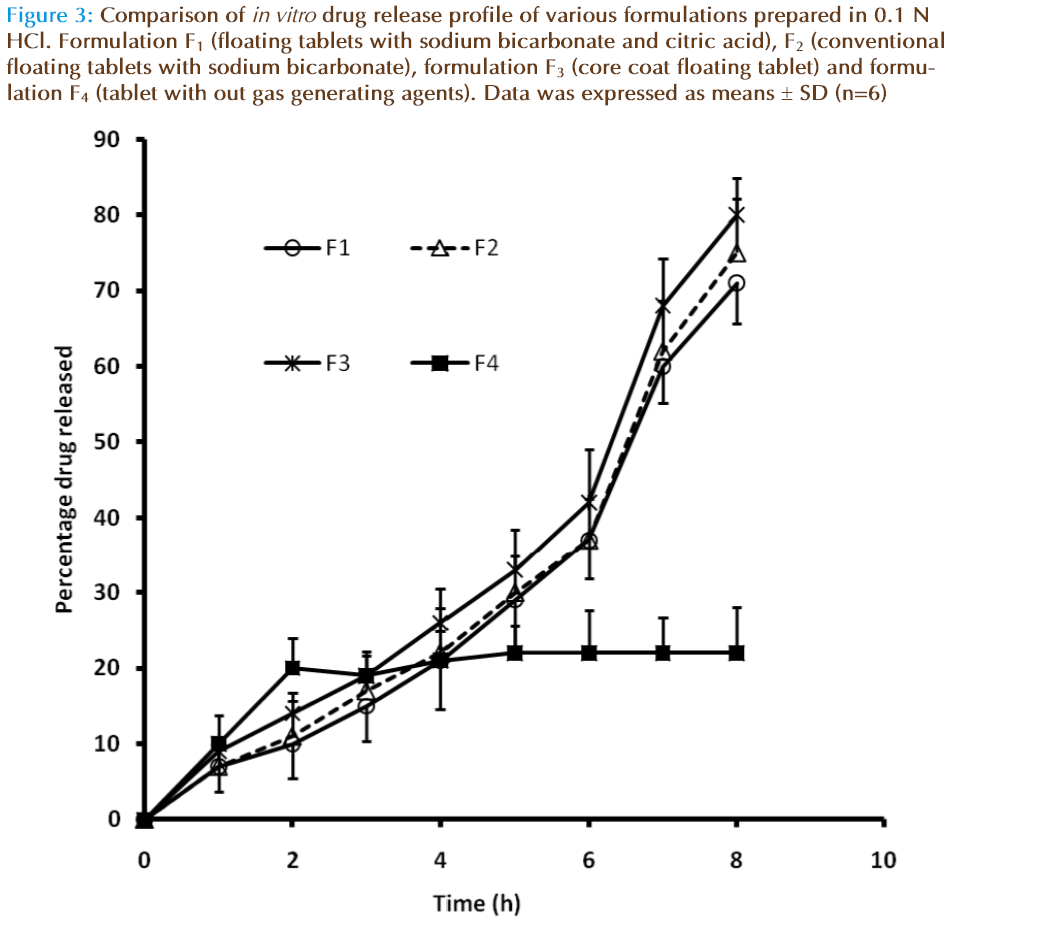
Figure 3: Comparison of in vitro drug release profile of various formulations prepared in 0.1 N HCl. Formulation F1 (floating tablets with sodium bicarbonate and citric acid), F2 (conventional floating tablets with sodium bicarbonate), formulation F3 (core coat floating tablet) and formulation F4 (tablet with out gas generating agents). Data was expressed as means ± SD (n=6)
Extended floating times can be achieved with an impermeable and insoluble coating such as rosin. This is necessary to retain its porosity; otherwise air entrapped will be slowly replaced with the release medium. Rosin and its derivatives has been extensively investigated as matrix forming agents, coating materials, and chewing gums [20–22]. Earlier we have used rosin as an insoluble hydrophobic matrix system for sustained release of diltiazem for more than 8 h [23]. In the current study, low molecular weight polyethylene glycol (MW 400) was incorporated with rosin as plasticizer to improve the mechanical properties and to provide suitable fl exibility to the coating polymer. The sub coated core was further film coated with low viscosity HPMC E15 to provide moisture protection and better adhesion between core and drug loading matrix. This also acts as cushioning agent which will protect the polymer (rosin) fracture during compression process [24]. The transverse section of tablets when observed has indicated that the core and coat were intact after the compression process. The prepared formulations have the size of 12 mm diameter, which is much higher than the tablet size (7 mm diameter) that could exit from the fed stomach regardless of its emptying pattern [25]. It was also reported that the non-disintegrating systems of a size in excess of the mean diameter of the pylorus (12.8 ± 7 mm) appear to be retained in the stomach for as long as the digestive phase is maintained [26]. Hence, it is assumed that the prepared tablets are likely to retain in the stomach until it disintegrates.
Cisapiride is a water insoluble drug; its release from the matrix is largely dependent on the erosion of the drug loading section. It was found that since the amount of HPMC in the drug-loading section is same, the drug release pattern is similar in all formulations. This was earlier reported by Zhenping et.al [27]. As to the type of the In vitro release, all profiles fi t either zero order or Higuchi type except F4 as given in Table 3. The In vitro dissolution of the conventional slow release tablet F4 was less compared with the floating layer tablets. The dissolution results are given in Figure 2.The release rate of F4 was reduced after replacement of simulated gastric fluid (SGF) with simulated intestinal fluid (SIF) after 2 h. The solubility of cisapride is pH dependent. Higher the pH, lower the solubility. The consequence is that the solubility of the drug and hence the dissolution of a formulation containing cisapride will decrease in SIF. This could results in significant decrease in absorption from upper gastrointestinal region and reduced bioavailability.
An inherent problem with hydrogel based matrix FDDS is the gradual loss of carbon dioxide bubbles and sustainability of floating system. The HPMC polymers with viscosity above 100 cps could form a stable hydrogel layer upon contacting with water. Another potential problem associated with gas generating FDDS is that the tablet could rapidly exit the stomach before becoming buoyant since the device cannot float immediately after administration and its buoyancy will be infl uenced by fl uctuations in the pH of the gastric fluids.
Conclusion
Stable, reliable, and persistent buoyancy was achieved by entrapping air inside an inert core and maintaining the porosity by an impermeable, water insoluble coating. This study showed that there is a potential for this novel intragastric, floating, core-coat tablet to remain in the stomach for a longer period of time and to have a better in vivo drug release compared with the conventional slow release tablet. Moreover, the regulation of drug release kinetics could be entirely done by suitable manipulation of polymers in the drug layer. Stable c ore will support the external drug layer even with the gradual or complete loss of gas generating system. The calculated regression coefficients showed higher r2 value with Higuchi models and zero order (F1, F2 and F3), except formulation F4 (Table 3). However, the regression values were found to be low with fi rst order kinetics. Thus, both zero-order and Higuchi models could be applicable. Since cisapiride has better solubility in SGF than in SIF, an improved bioavailability may be obtained when it is incorporated in this gastric retention delivery system. This novel FDDS could be used as a general model for the design of other gastric retention systems.
Conflict Of Interest
There are no confl icts of interest.
References
- Wang J, Tabata Y, Bi D, Morimoto K. Evaluation of mucoadhesive properties of animated gelatin microsphere. J Control Release 2001; 73: 223–31.
- Sheth PR, Tossounian JL. The hydrodynamically balanced system (HBSTM): a novel drug delivery system for oral use. Drug Dev Ind Pharm 1984; 10:313–39.
- Li S, Lin S, Daggy BP, Haresh LM, et al. Effect of formulation variables on the floating variables on the floating properties of gastric floating drug delivery system: Drug Dev Ind Pharm 2002; 28:783–93.
- Baumgartner S, Kristl J, Vrecer F, et al. Optimisation of floating matrix and evaluation of their gastric residence time. Int J Pharm 2000; 195: 125–35.
- Swati CJ,Amit JA,Sudhir VP,Kuchekar BS,et al.Formulation and evaluation of gastroretentive delivery system of propranolol hydrochloride. AAPS PharmSciTech 2009;10:1071–79.
- Muralidhar N,Chandrasekhar RG,Prabhakar RV,et al. Formulation and evaluation of gastroretentive delivery system of clarithromycin. AAPS PharmSciTech 2008; 9:231–37.
- Srivastava AK, Wadhwa S, Ridhurka D, et al. Oral sustained delivery of atenolol from floating matrix tablets-formulation and In vitro evaluation. Drug Dev Ind Pharm 2005; 31: 367–74.
- Xiaoqiang V, Minjie S, Feng Z, et al. Floating matrix dosage form for phenoporlamine hydrochloride based on gas forming agent: In vitro and in vivo evaluation in healthy volunteers. Int J Pharm 2006; 310:139–45.
- Ozdemir N, Ordu S, Ozkan Y. Studies of floating dosage forms of furosemide: In vitro and in vivo evaluations bilayer tablet formulations. Drug Dev Ind Pharm 2000; 26: 857–866.
- Krogel I, Bodmeier R. Development of a multifunctional drug delivery system surrounded by an impermeable cylinder. J Control Release 1999; 61: 43–50.
- Muller W, Anders E. Floating system for oral therapy.WO Patent 89,069, 56.March 28, 1989.
- Sunil KJ,Govind GP,Jain NK.Evalaution of porous carrier based floating orlistat microsphere for gastric delivery. AAPS PharmSciTech 2006;7:E1–E9.
- Timmermans J, Moes AJ. How well do floating dosage forms float? Int J Pharm 1990; 62: 207–216.
- Sandra S, Hendrik M, Karsten M. Characterization of poly (vinyl acetate) based floating matrix tablet. J Control Release 2008; 126: 149–55.
- Preechagoon Y,Charles BG. Analysis of cisapride in neonatal plasma using high-performance liquid chromatography with a base-stable column and fl uorescence detection. Journal of Chromatography B: Biomed. Sci. and Appl 1995; 670: 139–43.
- Hayashi T, Kanbe H, Okada M, et al. Formulation study and drug release mechanism of a new theophylline sustained release preparation. Int J Pharm 2005; 304: 91–101.
- Satturwar PM, Fulzule SV, Dorle AK. Biodegradation and in vivo biocampatability of rosin: A novel film forming polymer. AAPS Pharm Sci Tech 2003; 4: Article 55.
- Seymour RB, Carraher Jr, Charles E. In: Naturally occuring polymers- Polymer Chemistry; Seymour R.B. Charles E. Carraher, Jr. Ed; Marcel Dekker Inc: New York, 1992; Vol.1, 155–99.
- Raymond CR, Paul JS, Sian CO. Handbook of Pharmaceutical Excipents; 5th ed.; Pharmaceutical Press:Great Britain, 2006.
- Pathak YV,Nikore RL, Dorle AK. Study of rosin glycerol esters as microencapsulating materials. J Microencapsul 1985; 2: 137–140.
- Mandagade PM, Satturwar PM, Fulzule SV, et al. Rosin derivatives: novel film forming materials for controlled drug delivery.Rect Fun Poly 2002; 50: 233–242.
- Satturwar PM, Fulzule SV, Panyam J, et al. Evaluation of new rosin derivatives for pharmaceutical coating. Int J Pharm 2004; 270:27–36.
- Jacob S, Shirwaikar AA, Grover V. Formulation and evaluation of sustained release tablets using an insoluble rosin matrix system. Ind J Pharm Sci 2005; 67: 80–83.
- Chambin O, Rota MH, Rochat GH, et al. Performance of multilayered particles: infl uence of a thin Cushioning layer. Drug Dev Ind Pharm 2005; 31: 739–46.
- Kinget R, Kalala W, Vervoort L, Mooter GV. Colonic drug targeting. J Drug Target 1988; 6: 129–149.
- Davis SS, Hardy GJ, Taylor MJ, et al. The effect of food on the gastrointestinal transit of pellets and an osmotic device (Osmet). Int J Pharm 1984; 21: 331–340.
- Zhenping W,Zhanfeng Y,Dainzhou B. Design and evaluation of a two layer floating tablet for gastric retension using cisapiride as a model drug. Drug Dev Ind Pharm 2001; 27: 469–74.