Current Regulatory and Pharmacopoeial Status of Mycoplasma Testing in Concern with Vaccines Safety for Human Use
Received: 02-Jun-2021 Accepted Date: Jun 17, 2021 ; Published: 30-Jun-2021
Citation: Kalaivani M, Chaudhary P, Goyal M, et al. Current Regulatory and Pharmacopoeial Status of Mycoplasma Testing in Concern with Vaccines Safety for Human Use. J Basic Clin Pharma. 2021;12:S4:001.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Mycoplasma contamination is well known serious microbial contamination that encountered during the production of biopharmaceutical products produced from cell culture. Contamination to this biopharmaceuticals represents potential risk for the health of patients. To minimize this risk monitoring of mycoplasma contamination is preferred during the manufacturing process in cell culture substrates. The current methods recommended by United States Pharmacopoeia, European Pharmacopoeia, British Pharmacopoeia and Indian Pharmacopoeia for mycoplasma testing of biologics involve cell culture methods and indicator cell culture method. The conventional methods require long time period assays (minimum 28 days) and skill for interpretation of the results which limits the use of these assays. The use of Nucleic Acid Amplification Test (NAT) technique provides advantages over conventional microbiological methods in terms of analytical output, sensitivity and turnover time. The present review provide the overview about the conventional technique used, and their limitations, use of nucleic acid based method in mycoplasma testing of biologics and cell substrates. This review also provide the information about current regulatory requirements as prescribed by US-FDA, European Medicines Agency (EMA), World Health Organization (WHO) and guidelines for mycoplasma testing in biologicals, worldwide.
Keywords
Mycoplasma, Biologicals, Mycoplasma testing, Indian Pharmacopoeia
Introduction
Mycoplasmas are the smallest (0.3-0.8 μm diameter) and simplest self- replicating prokaryotes without cell wall that can form recognizable colonies on cell-free media [1]. The genome size of mycoplasma as recorded is 600-1700 kb with a relatively low G+C content, ranging from 23 to 41% [2]. The absence of rigid cell wall and incapability of peptidoglycan synthesis make mycoplasmas susceptible to various antibiotics [3]. Mycoplasma includes more than 250 species as identified in humans, animals, plants, and arthropods. Mycoplasma species include M. pneumoniae, M.fermentans, M. genitalium, M. hyorhinis, M. urealytium, M. penetrans and M. pirum have been found to be pathogenic to humans [4,5].
Mycoplasma contamination commonly occurs during development and manufacturing process of biopharmaceutical products including vaccines, at primary cell culture stage in particular. Contamination during synthesis occur from various sources such as Media, sera or reagents, Laboratory personnel, Incubators, Liquid Nitrogen, Airborne particles and aerosols, and improper sealing of culture dishes [6]. The contamination of mycoplasma does not cause turbidly or affects cell line growth and production scale but its presence compromises the safety of vaccines. Mycoplasma contamination in vaccines may lead to health risk in patients as it affects haemopoietic system, cardiovascular system, musculoskeletal system, gastrointestinal system, cutaneous system and genitourinary system and economical risk to the manufacturers due to product recall [7-9]. Their ability to cause chromosomal rearrangements has led them as a cause of cancer or work as cofactors for a variety of conditions including arthritis, Crohn’s disease and acquired immunodeficiency syndrome. Despite the challenges, the cell cultures should be tested for Mycoplasma contamination to fulfil regulatory requirements. Worldwide, regulatory authorities World Health Organization, International Conference on Harmonization, U.S. Code of Federal Regulations, EMA, and Central Drugs Standard Control Organisation lay out the technical testimonials for the detection of Mycoplasma at various stages during production or manufacturing of live virus vaccines or inactivated virus vaccines [10-14].
Pharmacopoeias, book of quality standards for drugs and pharmaceuticals prescribes methods and limits for analysis of various parameters to confirm identity, purity and potency of pharmaceuticals. The methods recommended by Indian Pharmacopoeia (IP 2018), United States Pharmacopoeia (USP 43), European Pharmacopoeia (Ph. Eur.10.2), and Japanese Pharmacopoeia (JP XV, 14), for testing of absence of mycoplasma includes (i) culture method/Standard cell culture method and (ii) indicator cell culture method (iii) Nucleic acid amplification techniques [15-18]. Pharmacopoeial methods rely on Broth/agar and indicator cell line tests to detect all mycoplasma species present in contaminated cell substrates and virus stocks used to produce viral vaccines. However, main drawback with these tests is long testing period which is not suitable for cultures have short life span. Due to time-consumption and tediousness, various procedures based on nucleic acid markers of Mollicutes have been developed and proposed as potential alternatives to the current available methods [15-18]. In order to reduce the time required for mycoplasma testing, pharmacopoeias recently included and recommended Nucleic Acid Amplification Test (NAT) as an alternative to either method for determination of absence of mycoplasma. From that prospective, the current review emphasizes on overview of mycoplasma testing methods procedure and present scenario of regulatory and pharmacopoeial requirements for mycoplasma testing in vaccines for human use.
Mycoplasma Testing Methods
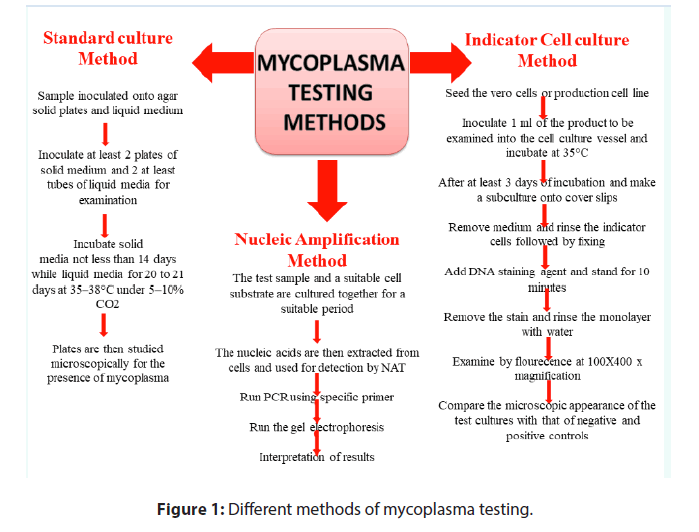
Several methods have been developed to detect mycoplasma contaminations in biopharmaceuticals production among which cell culture method and indicator cell culture method are the reference methods which are adopted in regulatory consideration. Recently, test for mycoplasma by using NAT method is also recommended as alternative method to standard culture method and indicator cell culture method (Figure 1) [15-18].
Standard culture method
Traditionally, the culture method is used to detect the mycoplasma in test sample in which both solid medium and liquid enrichment medium with phenol red dye are used and inoculated with test sample. The pH of media is balanced and incubated for 20-21 days at 35- 37°C under 5%-10% CO2 and nitrogen environment. At specific time intervals small aliquots of the enrichment culture are sub-cultured and incubated. After the incubation period the plates are observed under microscope for the presence of mycoplasma which should comply with limits as defined under pharmacopoeias. The test is invalid if one or more of positive controls do not show the growth of mycoplasma on at least one sub-culture plate. The test is invalid if one or more of the negative control show growth of mycoplasma.
Indicator cell culture method
Detection of mycoplasma in vaccine samples can also be done by indicator cell culture method in which cells are stained with a fluorescent dye that binds to their DNA. The indicator cell culture is inoculated with mycoplasma is considered as positive control while non-inoculated cell culture is used as negative control. Both the culture plates are incubated at 35-38°C in CO2 incubator until appropriate cell colonies appear on the plate. After incubation, the culture plates are fixed and stained with a DNA-binding fluorescent dye and analysed under a fluorescence microscope. The cells show positive test show fluorescent spot where as negative control not show appropriate fluorescence spot.
Nucleic Acid Amplification Test (NAT)
NAT is used as an alternative to culture-based methods to detect mycoplasma contamination of cell cultures. NAT has gained popularity due to assay sensitivity, simplicity, easy to use with a quick turnaround time. NAT used for detection of mycoplasmas by culturing test sample and suitable cell substrate together for a suitable period and then nucleic acid are extracted from the cells and supernatant. Further, amplification of extracted nucleic acids with specific primers is done that reveal the presence of the target nucleic acid sequence on comparison with international standard.
Pharmacopeial Specifications for Testing of Mycoplasma
Indian Pharmacopoeia, United States pharmacopoeia, British Pharmacopoeia, European Pharmacopoeia, and Japanese Pharmacopoeia recommend mycoplasma testing to confirm its absence of in final preparation [15-19]. Cell culture method and indicator cell culture method are the reference methods and are considered for approval of biopharmaceuticals production including vaccines [15-18,20]. However, these methods have their own limitations as they require expertise, time consuming and not able to detect all mycoplasma infections in cell cultures due to either specificity or sensitivity deficiencies. The emergence of advanced methods for detecting mycoplasma provides great opportunity to reduce testing time limits. Nucleic acid amplification test method is being extensively used to detect mycoplasma contamination in biotechnological derived medicinal products and vaccines [21,22]. The current challenges to the pharmaceutical industries and regulatory agencies are validating and determining the applicability of these methods to make sure that the products are free from any mycoplasma contamination generated during manufacturing process. Mycoplasma contamination even at very low levels is a serious problem in manufacturing of viral vaccines and biological [23,24]. In US Pharmacopoeia version 43 Chapter <63> Mycoplasma Tests (USP<63>) prescribes mycoplasma screening at the various stages of production including cell banking, virus seed stock preparation, unprocessed bulk harvesting, raw material uses and final product using culture methods [16]. Similarly, BP 2020 Appendix XVI B prescribes general chapter ‘Test for absence of mycoplasma’ for a master cell bank, working cell bank, virus seed lot or control cells, both the culture method and the indicator cell culture method are used [19]. NAT may be used as an alternative to one or both the methods after suitable validation and verification [25-30]. Current status of mycoplasma testing in various pharmacopoeias in vaccines for human use is given in Table 1.
| S. No. | Vaccines | BP 2020 | USP-43 | Ph.Eur. 10.2 | IP 2018 | WHO Recommendation |
|---|---|---|---|---|---|---|
| 1 | Measles and Rubella Vaccine (Live) | Monograph not present | Final lot | Serum used for propagation of cells, Trypsin used for preparing cell culture, single Harvest, Final Bulk, Final Lot. | ||
| 2 | Measles Vaccine (Live) | Test not present | Monograph not present | Test not present | Seed lot, Propagation and Harvest, Final Lot | Serum used for propagation of cells, Trypsin used for preparing cell culture, single Harvest, Final Bulk, Final Lot. |
| 3 | Measles Mumps Rubella Vaccine (Live) | Test not present | Monograph not present | Test not present | Test not present | Serum used for propagation of cells, Trypsin used for preparing cell culture, single Harvest, Final Bulk, Final Lot. |
| 4 | Mumps Vaccine (Live) | Test not present | Monograph not present | Test not present | Seed lot, Propagation and Harvest, Final Lot | Serum used for propagation of cells, Trypsin used for preparing cell culture, single Harvest, Final Bulk, Final Lot. |
| 5 | Poliomyelitis Vaccine, Live (Oral) | Test not present | Monograph not present | Test not present | Propagation and Harvest | Cell culture, Bovine or porcine trypsin, single harvest. |
| 6 | Poliomyelitis Vaccine (Inactivated) | Single Harvest | Monograph not present | Single Harvest | Propagation and Harvest | Cell Culture, Master working seed lot, Single harvest. |
| 7 | Rotavirus Vaccine (Live Attenuated, Oral) | Test not present | Monograph not present | Test not present | Virus Seed Lot, Virus Propagation and Harvest | Cell culture, Virus seed lot, control of vaccine production, single harvest and monovalent virus pools. |
| 8 | Rubella Vaccine (Live) | Test not present | Monograph not present | Test not present | Seed lot, Propagation and Harvest, Final Lot | Serum used for propagation of cells, Trypsin used for preparing cell culture, single Harvest, Final Bulk, Final Lot. |
| 9 | Varicella Vaccine, Live | Test not present | Monograph not present | Test not present | Test not present | Serum, Trypsin, Single Harvest, Final Lot. [30] |
| 10 | Yellow Fever Vaccine (Live) | Single harvest or Pool of single harvest | Monograph not present | Single harvest or Pool of single harvest | Seed lot, Propagation and Harvest | Master seed lot, working seed lot, single harvest. [31] |
| 11 | Influenza Vaccine (Human, Live , Attenuated) | Test not present | Monograph not present | Test not present | Propagation and Harvest, Seed lot, Monovalent Virus pool | Donor strains, master seed lot, working seed lot, master cell bank, working cell bank and production cell cultures. [32] |
| 12 | Japanese Encephalitis Vaccine (Human) | Monograph not present | Seed Lot, Propagation and Harvest | Cell culture, Cell culture medium, Master seed lot, Working seed lot. [33] | ||
| 13 | Japanese Encephalitis Vaccine Inactivated (Adsorbed, Human) | Monograph not present | Seed Lot, Propagation and Harvest | Cell culture, Cell culture medium, Master seed lot, Working seed lot. [33] | ||
| 14 | Japanese Encephalitis Live vaccine (Human) | Monograph not present | Propagation and Harvest | Master virus seed lot.[34] | ||
| 15 | Inactivated Hepatitis A Vaccine (Adsorbed) | Single Harvest | Monograph not present | Single Harvest | Propagation and Harvest | Single harvest, final bulk.[35] |
| 16 | Hepatitis A (Inactivated) and Hepatitis B (rDNA) Vaccine (Adsorbed) | Test not present | Monograph not present | Test not present | Test not present | Cell culture Medium, Single Harvest.[36] |
| 17 | Inactivated Hepatitis B Vaccine | Monograph not present | Propagation and Harvest | Single harvest or single harvests pool. [36] | ||
| 18 | Inactivated Influenza Vaccine (Split Virion) | Virus Seed Lot | Monograph not present | Virus Seed Lot | Seed lot, Propagation and Harvest | Cell culture, Trypsin. [37] |
| 19 | Inactivated Influenza Vaccine (Surface Antigen) | Virus Seed Lot | Monograph not present | Virus Seed Lot | Seed lot, Propagation and Harvest | Cell culture, Trypsin. [37] |
| 20 | Inactivated Influenza Vaccine (Whole Virion) | Virus Seed Lot | Monograph not present | Virus Seed Lot | Seed lot, Propagation and Harvest | Cell culture, Trypsin. [37] |
| 21 | Rabies Vaccine, Human | Test not present | Monograph not present | Test not present | Seed Lot, Propagation and Harvest | Serum, Trypsin, Primary hamster kidney cells, Virus seed lot, Single virus harvest. [29] |
| 22 | Tick-borne Encephalitis Vaccine (Inactivated) | Virus propagation and Harvest | Monograph not present | Virus propagation and Harvest | Seed lot, Propagation & Harvest | Cell culture medium, Master seed lot, Working seed lot, single cell harvest.[38] |
| 24 | Hepatitis B vaccine (rDNA) | Monograph not present | Test not present | Serum, Mycoplasma, Single harvest.[39] |
Table 1: Status of mycoplasma testing in vaccines for human use in BP 2020, USP-43, Ph.Eur.10.2 and IP-2018.
In European Pharmacopoeia 10.2, General chapter 2.6.7 prescribes either culture (broth and agar both) method and indicator cell culture method may be used to test mycoplasma to at stages of master cell bank, a working cell bank, a virus seed lot or for control cells [17]. Culture method alone is recommended to screen virus harvest or a bulk vaccine or final lot. It also states that NAT may be used as an alternative to either method after suitable validation and verification [17]. In harmonization with WHO, Indian Pharmacopoeia version 2018 prescribed general chapter 2.7.4 standard culture method and/or indicator cell culture method may be used for the absence of Mycoplasma for master cell bank, a working cell bank, and a virus seed lot. While standard culture method is recommended for virus harvests, bulk vaccine and final lot (batch). Further, Nucleic Acid Amplification test or any other method can also be used after suitable validation and approval by National Regulatory Authority [31-39].
Current Regulatory Requirements for Absence of Mycoplasma in Vaccines for Human Use
Mycoplasma contaminated vaccines cause serious adverse effects particularly in paediatric, geriatric, or immunocompromised patients and have multitudinous effects on the cell culture of biological products including vaccines [40]. Mycoplasma contamination of cell cultures during manufacturing process of vaccines were identified in 1950s raised the concern on safety of the product [41]. To maintain the quality of products and to minimize the health risk United States Public Health Service (US-PHS) established a test for detection of mycoplasma for viral vaccines produced in cell cultures in 1962 [42]. The US-FDA recommends testing of conventional mycoplasma in broth and agar media. Additionally tissue culture (indicator cell) assay was included i.e., “Points to Consider (PTC) in the characterization of cell lines used to produce biologicals” published by US-FDA in 1987 and updated in 1993 [43]. International Conference on Harmonisation, US-FDA and European Directorate for the Quality of Medicines (EDQM) issued various guidelines to detect and control presence of mycoplasma during in-process manufacturing and final lot (Table 2), [10, 44-46].
| Parameters | WHO recommendation [10] | US FDA guidelines [12] | EMA guidelines [17] | CDSCO guidelines [14] |
|---|---|---|---|---|
| Test method | Standard Culture method | Agar/ broth culture method | Agar/ broth culture method | Cell culture method |
| Indicator cell-culture method | Indicator cell culture method | Indicator cell culture method | Indicator cell culture method | |
| NAT based methods | PCR-based assays | Nucleic Acid amplification Techniques | ||
| Mycoplasma testing at Stages of production | Master Cell bank | Master Cell bank | Master seed, | Master Cell bank |
| Working cell bank | Working cell bank | master cell seed (stocks) | Working cell bank | |
| Virus seed | Virus seed harvest | working seed | Virus seed lots | |
| Single harvest | End-of-production cells | Cell lots | Control cells | |
| Virus seed final | Virus harvest | |||
| Vaccine harvest | Bulk vaccine | |||
| Final lot | ||||
| Reference Strains used | Acholeplasma laidlawii, | Mycoplasma pneumonia | Achdeplasma laidlawaii | Achdeplasma laidlawaii |
| Mycoplasma fermentans | Mycoplasma orale | Mycoplasma hyorhinis | Mycoplasma gallisepticum | |
| Mycoplasma orale | Mycoplasma hyorhinis | Mycoplasma synoviae | Mycoplasma hyorhinis | |
| Mycoplasma pneumonia | Mycoplasma orale | Mycoplasma synoviae | ||
| Mycoplasma fermentans | Mycoplasma orale | |||
| Mycoplasma pneumoniae |
Table 2: Regulatory guidelines for mycoplasma testing issued by National Regulatory agencies.
US-FDA requires mycoplasma testing where cell culture is used for manufacture/production of live viral vaccines and inactivated viral vaccines to ensure that these vaccines are free from mycoplasma. US- FDA also provides guidance for qualification of cell substrates used in the production of viral vaccines and recommends that master cell bank, master viral seed and biological raw materials such as serum should be free from mycoplasma. US-FDA recommends testing for mycoplasma at various stages of production starting from pre-filtered harvest or post-production cells stage till final lot [47]. Broth/agar and indicator cell line methods were developed to detect all mycoplasma species isolated from contaminated cell substrates, viral vaccines, and virus stock collection. As described earlier these tests are times consuming, the products which have short shelf-life require rapid testing. For this reason, alternative tests are routinely accepted by the regulatory bodies, as long as they are well verified and validated. Recently, US- FDA repudiate regulation of use of specified test for the presence of Mycoplasma for live virus vaccines and inactivated virus vaccines produced from such living cell cultures. Removal of specified test for absence of Mycoplasma provides pliability for adopting new and evolving justified scientific methods without risking public health [48].
WHO provides various technical report series (TRS) for manufacturing and quality control of vaccines. These guidelines are adopted by National regulatory authorities (NRAs) and manufacturers to ensure the quality of essential vaccines in a global market. WHO also update the technical report series to provide guidance for regulation and licensing of various types of vaccines. The TRS include recommendations for individual vaccines, which prescribed the mycoplasma testing on various production stages including master cell bank, working cell bank, and virus seed lot (or bank) by either using standard cultures or the indicator cell culture method [49]. The WHO also recommends use of NAT to detect mycoplasma in live and inactivated viral vaccines. WHO established first international standard for mycoplasma DNA and nucleic acid amplification technique-based assays designed for mycoplasma detection with a potency limit of 200,000 IU/ml. The standard and the defined limit should be used as reference preparation for characterization of NAT assays and for calibration of quantitative assays as in a common unit to define regulatory requirements.
In India, for preparation of the Quality Information for Drug Submission for New Drug Approval: Biotechnological/Biological Products, National Regulatory authority Central Drugs Standard Control Organisation (CDCSO) issued ‘Guidance for industry’ which states that to consider Pharmacopoeial methods for mycoplasma testing at all stages of production i.e., master cell bank, working cell bank, virus seed lots, control cells, virus harvests, bulk vaccine and final lot in harmonization with other regulatory authorities.
Conclusion
Raw materials are required to be cautiously screened before and at every stage of vaccine production can prevent mycoplasma contamination in vaccines. The broth/agar culture method is very sensitive that should be carefully controlled; NAT is preferred in industry due to its sensitivity in detection of mycoplasma. NAT provide manufacturer perfection and credence in results required in production and batch release of final products. 21 CFR 610.30, USP<63>, FDA guidance, EMA guidance, CDSCO provide guidelines for quality and use of validated testing methods for mycoplasma testing. Further, they not only provide streamlined validated testing procedures but also give the flexibility to manufacturer to select the most appropriate method depends on the specific biological product.
Finally, in the era of biotechnologically derived therapeutics (monoclonal antibodies, genetically-engineered pharmaceuticals and vaccines) determination of mycoplasma contamination in vaccines during production or at final stage needs to be considered of utmost importance. Recently regulatory agencies and pharmacopoeias recommended suitable fastest methods like NAT based or ELISA based for detection of mycoplasma and also prescribed validation parameters and other requirements in adopting these methods. The current challenges/issues to the pharmaceutical industries and regulatory agencies are to select the suitable mycoplasma testing among to make sure the products are free from any mycoplasma contamination. However, alternative methods can be developed and adopted only after their successful qualification and validation in comparison to existing compendial methods and proper authorization by the competent authority.
Conflict of Interest
None declared.
REFERENCES
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiology and Molecular Biology Reviews. 1998;62(4):1094-1156.
- Rottem S, Barile MF. Beware of mycoplasmas. Trends in Biotechnology. 1993;11(4):143-151.
- Chandler DKF, Volokhov DV, Chizhikov VE. Historical Overview of Mycoplasma Testing for Production of Biologics. American Pharmaceutical Rev. 2011.
- He J, Liu M, Ye Z, et al. Insights into the pathogenesis of Mycoplasma pneumoniae (Review). Molecular Medicine Reports. 2016;14(5):4030-4036.
- RMC. Mycoplasma pneumoniae: Proposed nomenclature for atypical pneumonia organism (Eaton agent). Science. 1963;140(3567):662-667.
- Nikfarjam L, Farzaneh P. Prevention and detection of Mycoplasma contamination in cell culture. Cell Journal. 2012;13(4):203.
- Zinöcker S, Wang MY, Gaustad P, et al. Mycoplasma contamination revisited: mesenchymal stromal cells harboring Mycoplasma hyorhinis potently inhibit lymphocyte proliferation in vitro. PloS One. 2011;6(1):e16005.
- Fong IW. New perspectives of infections in cardiovascular disease. Current cardiology reviews. 2009; 5(2):87-104.
- Davies HD. Committee on Infectious Diseases. Infectious complications with the use of biologic response modifiers in infants and children. Pediatrics. 2016;138(2).
- Recommendations for the preparation, characterization and establishment of international and otherbiological reference standards. World Health Organization. 2006.
- Draft Guideline on Viral Safety Evaluation of Biotechnology Products Derived From Cell Lines of Human or Animal Origin; Availability Fed Regist. 1998;63(185):21882-21891.
- Points to consider in the characterization of celllinesused to producebiologicals. U.S. Food and Drug Administration. 1993.
- VICH GL34: Biologicals: testing for the detection of Mycoplasma contamination. European Medicines Agency. 2013.
- Guidance for Industry on Submission of Clinical Trial Application for Evaluating Safety and Efficacy. Central Drugs Standards Control organization. 1945.
- Mycoplasma tests Chapter 2.7.4. Indian Pharmacopoeia. 2018.
- United States Pharmacopeia. Mycoplasma Tests. 2020.
- European Pharmacopoeia Chapter 2. Mycoplasmas. 2020.
- Japanese Pharmacopoeia. Mycoplasma Testing for Cell Substratesused for the Production of Biotechnological/BiologicalProducts.
- British Pharmacopoeia. Test for Absence of Mycoplasma. 2020.
- Garner CM, Hubbold LM, Chakraborti PR. In vitro testing of platinum-based drugs on a panel of human ovarian tumour cell lines. 2001;59(1):295.
- Loens K, Ieven M. Mycoplasma pneumoniae: Current knowledge on nucleic acid amplification techniques and serological diagnostics. Frontiers in microbiology. 2016;7:448.
- Deutschmann SM, Kavermann H, Knack Y. Validation of a NAT-based Mycoplasma assay
- Chernov VM, Chernova OA, Sanchez-Vega JT. Mycoplasma contamination of cell cultures: Vesicular traffic in bacteria and control over infectious agents. Acta Naturae. 2014;6(3):1-22.
- David SA, Volokhov DV, Ye Z, Chizhikov V. Evaluation of Mycoplasma inactivation during production of biologics: egg-based viral vaccines as a model. Applied and Environmental Microbiology. 2010;76(9):2718-28.
- Requirements for measles, mumps and rubella vaccines and combined vaccine (live). WHO Tech RepSer.1994.
- Recommendations to assure the quality, safety and efficacy of poliomyelitis vaccines (oral, live, attenuated) .WHO Tech RepSer. 2014.
- WHO Expert Committee on Biological Standardization. Sixty-third Report. 2014.
- Recommendations to assure the quality, safety and efficacy of poliomyelitis vaccines (inactivated). WHO Tech RepSer.2015.
- WHO Expert Committee on BiologicalStandardization. WHO TRS 941. 2007.
- WHO Expert Committee on BiologicalStandardization. WHO TRS 848. 1993.
- Proposedamendments to the Requirements for Yellow Fever Vaccines. WHO Tech RepSer. 2008.
- WHO Expert Committee on Biological Standardization. World Heath Organisation. 2009.
- WHO Expert Committee on Biological Standardization. World Heath Organisation. 2007.
- WHO Expert Committee on Biological Standardization. Requirements for hepatitis A vaccine (Inactivated). WHO TRS 858 Annex 2.
- WHO Annex 4 Recommendations to assure the quality, safety and efficacy of recombinant hepatitis B vaccines Replacement of Annex 2 of WHO Technical Report Series. WHO Technical Report Series. 2013.
- Recommendations to assure the quality, safety and efficacy of recombinant hepatitis B vaccines. WHO Expert Committee on Biological Standardization.
- WHO Expert Committee on Biological Standardization. Recommendations for the production and control of influenza vaccine (Inactivated). 2005.
- WHO Expert Committee on Biological Standardization. World Heath Organisation. 2006.
- WHO Expert Committee on Biological Standardization. World Heath Organisation.2010.
- Zinöcker S, Wang MY, Gaustad P, et al. Mycoplasma contamination revisited: mesenchymal stromal cells harboring Mycoplasma hyorhinis potently inhibit lymphocyte proliferation in vitro. PLoS One. 2011;6(1):e16005.
- Chandler DKF, Volokhov DV, Chizhikov VE. Historical Overview of Mycoplasma Testing for Production of Biologics. Amer Pharma Rev. 2011.
- CFR - Code of Federal Regulations Title 21. 2010.
- Points to consider in the characterization of cell lines used to produce biologicals. Department of Health and Human Services. 1993.
- Guidance on viral safety evaluation of biotechnology products derived from cell lines of human or animal origin; availability. Fed Regist. 1998;63(185):51074-51084.
- VICH GL34: Biologicals: testing for the detection of Mycoplasma contamination. European Medicines Agency. 2013.
- Guidance for Industry on Submission of Clinical Trial Application for Evaluating Safety and Efficacy. Central Drugs Standards Control organization. 1945.
- David SA, Volokhov DV, Ye Z, et al. Evaluation of Mycoplasma inactivation during production of biologics: egg-based viral vaccines as a model. Applied and Environmental Microbiology. 2010;76(9):2718-2728.
- Revocation of the Test for Mycoplasma. Food and Drug Administration, Department of Health and Human services.
- Expert Committee on Biological Standardization. WHO Tech Rep Ser. 2014;987:1-42.