Causality assessment of adverse drug reaction in Pulmonology Department of a Tertiary Care Hospital
- *Corresponding Author:
- Dr. Amer Khan
Deccan School of Pharmacy, New Malakpet, Hyderabad, Telangana, India.
E-mail: amer.pharmd@gmail.com
Abstract
Background: Adverse drug reaction (ADR) is considered to be the sixth leading cause of death. The incidence rate estimates approximately 2% of hospital admissions are due to ADRs. Objective: To monitor ADRs in Pulmonology department of a tertiary care hospital patient with pulmonary diseases in an inpatient department of pulmonology. Materials and Methods: A prospective, single centered, observational and open labeled study was carried out in Princess Esra Hospital. The patient population was broadly divided into four categories based on diagnosis - chronic obstructive pulmonary disease, Infections, Asthma and Others. Suspected ADRs were reported, analyzed, and causality assessment was carried out using Naranjo’s algorithm scale. Results: A total of 302 patients were observed, of which 98 patients experienced ADRs, which accounted for 32.23% of the incidence and totally 160 ADEs were observed. Adult Patients were found to have higher incidence (32.09%) while the incidence rate was slightly greater in geriatric patients (32.39%). The highest incidence of ADEs were found in others group (78.57%). Majority of ADRs were suspected to be due to theophylline (19.39%). Gastrointestinal system (38.75%) was the most common organ system affected due to ADRs. Drug was withdrawn in 12 patients, and specific treatment was administered to 32 patients in view of clinical status. Specific treatment for the management of suspected reaction was administered in 32.65% of ADR reports. Conclusion: A relatively high incidence of adverse drug events (32.2%) have been recorded which shows that not only Geriatric patients, but also adults are more susceptible to adverse drug effects. A number of drugs in combination were used, and ADEs often get multiplied. Careful therapeutic monitoring and dose individualization is necessary.
Keywords
Adverse drug reactions, Assessment Management, causality, Naranjo algorithm, pulmonology
Introduction
Adverse drug reactions (ADRs) are known perils of drug therapy. An ADR may be simply defined is as an undesirable effect of a drug apart from its expected therapeutic action transpiring during clinical use.[1] Adverse reactions occurring due to drug usage may involve Allergies, toxicities, and side effects. An allergy is a hypersensitivity reaction to a drug. Majority of them are IgE-mediated and usually arise immediately after drug administration. For example early-onset urticaria, anaphylaxis, brochospasm, erythema multiforme, Stevens-Johnson syndrome, etc., Toxicity occurs when drugs are administered in quantities larger than that can be physiologically managed by the host. This mainly results from either immoderate dosing or impaired drug metabolism. Examples of toxicity include penicillin related neurotoxicity (e.g. twitching, seizures) and the toxicities caused by aminoglycosides. Side-effects include ADRs that are neither immunological nor related to toxic levels of drug. An example of this is dyspepsia induced by erythromycin.[2] A side-effect may be defined as an expected and known effect of a drug, which is unrelated to the intended therapeutic action. The term “side-effect” tends to normalize the notion of injury from drugs. It is recommended that this term should be avoided in favor of ADR.[3]
According to World Health Organization, ADR is defined as a response to a drug which is noxious and un-intended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function. It is also defined as an undesirable effect, reasonably associated with the use of the drug that may occur as a part of the pharmacological action of a drug or may be unpredictable in its occurrence.[4,5]
Adverse drug reaction is considered to be the sixth leading cause of death. The incidence rate estimates approximately 2% of hospital admissions are due to ADRs. Drug-attributed deaths are estimated to be 0.17% in all medical inpatients. About 0.40% of ADRs identified were directly linked to high costs. ADRs not only increase the mortality and morbidity but also multiply the health care cost.[6] ADR monitoring is primarily essential for drugs with narrow therapeutic index.[7-9] Theophylline has been used for many years for the treatment of asthma and chronic obstructive pulmonary disease (COPD). The incidence of ADRs due to theophylline has been found to be 4.71%, of which nausea, loss of appetite (anorexia) and palpitation were common.[10]
The study of ADRs is essential in order to determine the incidence of ADRs in medical inpatients, estimate the contribution of ADRs to hospital admissions, characterize the types of ADRs observed, determines predisposing risk factors and to estimate the costs of ADRs in terms of ADR-related excess hospital stay.[11]
Within this context, the aim of our research was to evaluate ADRs, by analyzing the clinical features as well as the frequency of ADRs, the role of drugs in such reactions occurring during the hospitalization in Pulmonology Department, Internal Medicine Department.
Materials and Methods
This study was conducted at Princess Esra Hospital, a group of Owaisi Hospitals, Hyderabad. It is a Tertiary Care Hospital with 1150 bed capacity. The study was single centered, prospective, observational and open labeled carried out for a period of 6 months from December 2013 to May 2014. Hospital approval was obtained from the medical superintendent before initiating the study. The patient selection was random and the patient population was divided into four broad categories based on diagnosis as:
• Chronic obstructive pulmonary disease
• Infections (pneumonia, tuberculosis (TB), lower respiratory track infection)
• Asthma
• Others (pleural effusion, anti-tubercular drug induced hepatitis, obstructive sleep apnea, interstitial lung disease, pleurisy, obesity hypoventilation syndrome, corpulmonale).
Verbal Informed consent (in the vernacular language) was sought from the patients before their enrollment, on the basis of inclusion and exclusion criteria. Patients of either gender above 18 years admitted into (Pulmonology Department) were included in the study. Pediatrics and pregnant patients were excluded from the study. During the study, patients were monitored from the day of admission till the day of discharge. Sources of data were case sheets and verbal information while counseling the patients. The details were collected in patient profile form designed for the study purpose. The details included: Demographics, medical history, medication history, laboratory data, history of drug allergy along with causative drug, current therapy, suspected ADR, description of ADR, date of onset, management and outcome aspects. Suspected ADRs were reported, analyzed and a causality assessment was carried out using Naranjo’s algorithm scale.
Results
During the study period, a total of 304 patients were monitored, of which 160 ADRs were observed in 98 patients accounting 32.23% of the incidence). Majority of the patients (n = 60) experienced one ADR, followed by 24 patients who suffered from two ADRs, eight patient experienced three ADRs, four patients experienced four ADRs, while two patient have experienced six ADRs. During the study, it was observed that each patient on an average experienced at least 1.63 ADRs. Based on sex, distribution of ADRs is shown in Table 1.
| Sex | Number of ADE | Number of patients with ADR |
|---|---|---|
| Male | 66 | 50 |
| Female | 94 | 48 |
| Total | 160 | 98 |
ADE: Adverse drug event, ADR: Adverse drug reaction
Table 1: Distribution of ADRs based on sex
Demographics and adverse drug reaction incidence
Among 154 male patients monitored, the incidence of ADRs was 32.5% (n = 50) in males, which were almost similar to 32% (n = 48) observed in females). 32.09% of adults experienced ADRs, which was slightly higher in geriatric patients (32.39%). The patients categorized in other group had the highest incidence of ADRs (78.57%) in contrast to 56.52% in infection group, 50% in asthmatic patients and 48.72% in COPD patients).
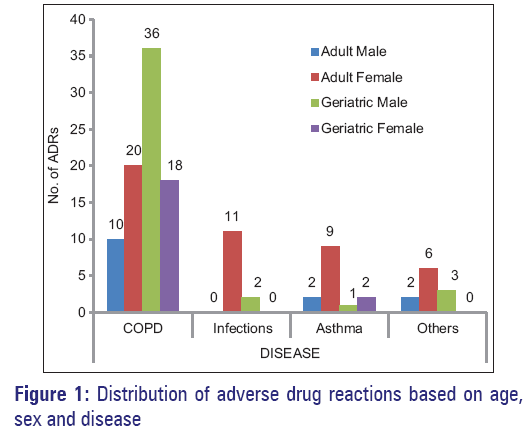
Female adults’ experienced highest (72 out of 160 events i.e. 45%) while least in male adults (18 out of 160 events, i.e. 11.25%). This high prevalence of ADRs could be attributed to multiple drug intakes, which was evident in our study as 14.78 drugs prescribed to patients irrespective of the age, gender and diagnosis). Distribution of ADRs Based on age, sex and disease, is shown in Figure 1.
Drugs implicated and organ system affected with adverse drug reactions
Drugs contributing majorly to ADRs were theophylline (19.39%), paracetamol (6.66%), salbutamol (5.45%) and levocetirizine (5.45%), respectively. Gastrointestinal system (38.75%) was the most common organ system affected due to ADR’s followed by a neurological system (22.5%), cardiovascular system (12.5%) as shown in the Table 2. Where-as Ceftazidine and Ranitidine showed highest prevalence rate of ADR. The detailed drug vs prevalence rate of ADR is shown in Table-3. Where-as Ceftazidine and Ranitidine showed highest prevalence rate of ADR. The detailed drug vs prevalence rate of ADR is shown in Table-3.
| Organ system | Frequency | Percentage |
|---|---|---|
| Gastrointestinal | 62 | 38.75 |
| Abdominal pain | 4 | |
| Anorexia | 6 | |
| Constipation | 14 | |
| Diarrhea | 8 | |
| Heart burn | 4 | |
| Hepatitis | 4 | |
| Nausea | 12 | |
| Vomiting | 10 | |
| Hematological | 6 | 3.75 |
| Anemia | 6 | |
| Respiratory | 2 | 1.25 |
| Cough | 2 | |
| Neurological | 36 | 22.5 |
| Dizziness | 4 | |
| Headache | 6 | |
| Insomnia | 12 | |
| Restlessness | 14 | |
| Cardiovascular | 20 | 12.5 |
| Hypertension | 4 | |
| Hypotension | 6 | |
| Orthostatic hypotension | 2 | |
| Palpitations | 4 | |
| Tachycardia | 4 | |
| Endocrinological | 14 | 8.75 |
| Hyperglycemia | 12 | |
| Hyperkalemia | 2 | |
| Dermatological | 8 | 5 |
| Pruritus | 6 | |
| Pain at injection site | 2 | |
| Others | 12 | 7.5 |
| Fatigue | 6 | |
| Oligurea | 2 | |
| Sweating | 2 | |
| Red colored urine | 2 |
ADR: Adverse drug reaction
Table 2: Distribution of ADRs in different systems of the body
| Drug | Number of times Frequency | Prevalence | |
|---|---|---|---|
| drug given | of ADR | (%) | |
| Pantoprazole | 282 | 12 | 0.04 |
| Clarithromycin | 14 | 4 | 0.29 |
| Cefoperazone+sulbactam | 98 | 4 | 0.04 |
| Levofloxacin | 6 | 4 | 0.67 |
| Isoniazid | 30 | 2 | 0.07 |
| Pyrazinamide | 30 | 4 | 0.13 |
| Furosemide | 74 | 14 | 0.19 |
| Levocetirizine | 202 | 18 | 0.09 |
| Ondansetron | 76 | 10 | 0.13 |
| Ursodiol | 6 | 2 | 0.33 |
| Chlorphinaramine maleate | 4 | 2 | 0.5 |
| Chlordiazepoxide | 4 | 2 | 0.5 |
| Piperacillin+tazobactam | 36 | 14 | 0.39 |
| Iron | 36 | 8 | 0.22 |
| Sucralfate | 56 | 4 | 0.07 |
| Paracetamol | 144 | 22 | 0.15 |
| ORS | 44 | 2 | 0.04 |
| Ceftazidine | 2 | 2 | 1 |
| Budesonide | 288 | 16 | 0.06 |
| Metronidazole | 22 | 8 | 0.36 |
| Linezolid | 4 | 2 | 0.5 |
| Zolpidem | 8 | 2 | 0.25 |
| Losartan | 8 | 2 | 0.25 |
| Prazosin | 6 | 2 | 0.33 |
| Amiodarone | 6 | 2 | 0.33 |
| Levothyroxine | 12 | 2 | 0.17 |
| Amlodipine | 44 | 8 | 0.18 |
| Atenolol | 14 | 2 | 0.14 |
| Salbutamol | 288 | 18 | 0.06 |
| Montelukast | 194 | 4 | 0.02 |
| Theophylline | 266 | 64 | 0.24 |
| Rabeprazole | 6 | 4 | 0.67 |
| Amoxicillin+clavulanate | 158 | 6 | 0.04 |
| Methyl prednisolone | 112 | 14 | 0.12 |
| Hydrocortisone | 110 | 8 | 0.07 |
| Moxifloxacin | 44 | 6 | 0.14 |
| Rosuvastatin | 8 | 2 | 0.25 |
| Terbutaline | 10 | 2 | 0.20 |
| Ethambutol | 30 | 2 | 0.07 |
| Torsemide | 16 | 2 | 0.12 |
| Butyl scopolamine | 18 | 2 | 0.11 |
| Diclofenac | 16 | 2 | 0.12 |
| HRZE | 30 | 4 | 0.13 |
| Metoprolol | 16 | 2 | 0.12 |
| Insulin | 90 | 2 | 0.02 |
| Rifampacin | 30 | 2 | 0.07 |
| Ranitidine | 2 | 2 | 1 |
| Furosemide+spironolactone | 8 | 2 | 0.25 |
| Tramadol | 44 | 4 | 0.09 |
ADR: Adverse drug reaction, ORS: Oral rehydration salt, HRZE: Isoniazid, rifampicin, pyrazinamide and ethambutol
Table 3: Prevalence of ADRs in the study
Management and outcome aspects of adverse drug reaction’s
Outof98 patientswithADRs, drugwaswithdrawn (de-challenged) in 12 patient (hypotension, tachycardia, palpitation, hepatitis, pruritis, hyperkalemia) and specific treatment was administered to 32 (abdominal pain-4, constipation-6, diarrhoe-6, anemia-4, insomnia-4, hypotension-2, pruritis-6) patients in view of clinical status. Full recovery was observed in 68 patients and rest of the patients had partial recovery. More-over the causative drug for 12.24% of ADRs were withdrawn owing the risk involved, which resulted in the recovery of 69 Specific treatment for the management of suspected reaction.38% patients. Four patients were re-challenged with the drug, which resulted in the reappearance of ADRs.
Specific treatment for the management of suspected reaction was administered in 32.65% of ADR reports.
Causality assessment of adverse drug reactions
Naranjo algorithm was used to assess the causality which revealed that ADRs can be categorized into 55% probable, 42.5% as possible and 2.5% of ADRs as definite which is shown in Table 4.
| Severity | Number of ADRs | Percentage of ADRs |
|---|---|---|
| Definite (>9) | 4 | 2.5 |
| Probable (5-8) | 88 | 55 |
| Possible (1-4) | 68 | 42.5 |
| Doubtful (0) | 0 | 0 |
ADR: Adverse drug reaction
Table 4: Severity-Assessment of ADRs
Severity assessment of adverse drug reactions
Severity assessment indicated that 51.25% (n = 82) of the suspected reactions were mild while 27.5% (n = 44) were moderate and 21.25% (n = 34) of them were severe in nature as shown in Table 4.
Discussion
Our study determines the incidence of ADR s in Pulmonology department and establishes the strategies to reduce and prevent the occurrence of ADRs. Such approaches will not only improve the quality of life of patients,’ but also minimize the cost associated with ADRs’ contingency.
Our finding discloses fact that the incidence of ADRs multiples with increase in number of drugs per prescription, which also has been highlighted by other previously published studies.[9] The prevalence of adverse drug events, in our study, was nearly 1.5 times higher than a similar study conducted by Tyagi et al.[10,11]
Of all the drugs used in Pulmonology Department, the highest incidence of ADRs was seen with the use of theophylline, which replicates the findings of study conducted by Ohta et al.[12] The gastrointestinal effects of theophylline can be minimized by consuming it with food. As theophylline has a narrow therapeutic index, it serum levels should be monitored to prevent theophylline associated cardio-toxicity.
Number of ADEs caused by anti-TB drugs in our study was similar to a previous study carried out by Yee et al. However, the figure was four times higher in a study by Gholami et al.[13,14] The contribution of antibiotics to ADRs was slightly less when compared to a study conducted by Gallelli et al.[12] Recovery after drug withdrawal in Gallelli et al. study was higher than our study. This may be due to a high certainity of drug-ADR relationship in their study.[11]
The study re-establishes that patients suffering from severe or acute respiratory disorders generally use multiple drugs and have increased susceptibility to ADRs and such patients should be carefully monitored to reduce ADRs associated morbidity. In most of the clinical settings, there is no proper reporting and monitoring of ADRs. Lack of formal pharmacovigilance centers is a major issue in developing countries responsible for under-reporting of ADRs. Establishment of pharmacovigilance centers with effective monitoring and reporting will play a significant role in preventing and managing the ADRs.
Limitations
• The study included only adult and geriatric population admitted in Pulmonolgy Department
• Although respiratory diseases like asthma and other RTIs are common in children, pediatric population was excluded owning difference in the biological and physiological make-up of children and also that pediatric patients were admitted in altogether different hospital, which was beyond the scope of the study
• Further larger studies involving all age groups may be helpful in rationalizing the drug therapy in respiratory diseases.
Conclusion
A relatively high incidence of adverse drug events (32.2%) have been recorded which shows that not only geriatric patients but also adults are more susceptible to adverse drug effects. A number of drugs in combination were used, and ADEs often get multiplied. Careful therapeutic monitoring and dose individualization is necessary. The incidence of ADRs was highest in geriatric patients. Nonetheless, adult patients also showed higher incidence, which could attribute to the use of multiple drugs administered, to minimize this high incidence of ADRs dose individualization and therapeutic monitoring of drugs is essential. Clinical studies to elicit the toxicodynamics of these ADEs and safety versus risk issues could be beneficial in devising strategies for its rational use in respiratory diseases.
Acknowledgements
We express our thanks to Dr. S.A AzeezBasha, Principal, Deccan School of Pharmacy for necessary facilities, helping and motivating us during the project work. We also thank Dr. Aleem (Pulmonologist, Esra Hospital) for providing valuable guidance and continuous encouragement.
References
- Shamna M, Dilip C, Ajmal M, Linu Mohan P, Shinu C, Jafer CP, et al. A prospective study on Adverse Drug Reactions of antibiotics in a tertiary care hospital. Saudi Pharm J 2014;22:303-8.
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: A clinician’s guide to terminology, documentation, and reporting. Ann Intern Med 2004;140:795-801.
- Granowitz EV, Brown RB. Antibiotic adverse reactions and drug interactions. Crit Care Clin 2008;24:421-42, xi.
- World Health Organization. Handbook of Resolutions and Decisions of the World Health Assembly and Executive Board WHA 16.36 Clinical and Pharmacological Evaluation of Drugs. Vol. 11948-1972. Geneva: World Health Organization; 1973.
- Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000;356:1255-9.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998;279:1200-5.
- Ohta K, Fukuchi Y, Grouse L, Mizutani R, Rabe KF, Rennard SI, et al. A prospective clinical study of theophylline safety in 3810 elderly with asthma or COPD. Respir Med 2004;98:1016-24.
- Newnham DM. Asthma medications and their potential adverse effects in the elderly: Recommendations for prescribing. Drug Saf 2001;24:1065-80.
- Khan FA, Nizamuddin S, Huda N, Mishra H. A prospective study otolaryngology department of a tertiary care hospital in North India. Int J Basic Clin Pharmacol 2013;2:548-53.
- Moore N, Lecointre D, Noblet C, Mabille M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol 1998;45:301-8.
- Tyagi N, Gulati K, Vijayan VK, Ray A. A study to monitor adverse drug reactions in patients of chronic obstructive pulmonary disease: Focus on theophylline. Indian J Chest Dis Allied Sci 2008;50:199-202.
- Gallelli L, Ferreri G, Colosimo M, Pirritano D, Guadagnino L, Pelaia G, et al. Adverse drug reactions to antibiotics observed in two pulmonology divisions of catanzaro, Italy: A six-year retrospective study. Pharmacol Res 2002;46:395-400.
- Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med 2003;167:1472-7.
- Gholami K, Kamali E, Hajiabdolbaghi M, Shalviri G. Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients. Pharm Pract (Granada) 2006;4:134-8.