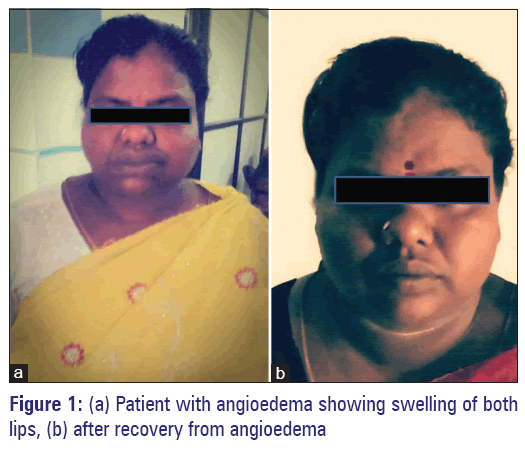
Carbamazepine-induced angioedema.
- *Corresponding Author:
- Dr. Ansha Subramanian
Department of Pharmacology, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India.
E-mail: go2ansha@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Angioedema is a rare adverse reaction of carbamazepine, which causes localized tissue edema in submucosal and subcutaneous tissue mediated by histamine, serotonin, and kinins (bradykinin). We report a case of 34‑year‑old female who developed angioedema, 24 h after administration of carbamazepine for treating bipolar disorder. Patient’s symptoms responded rapidly with antihistamine therapy and with the withdrawal of carbamazepine, the offending drug. Carbamazepine‑induced angioedema is a life‑threatening reaction which requires immediate treatment and monitoring in order to avoid morbidity and mortality.
sushihouse kolstads americanpridefasteners trueforge hotelposeidon youngswoodfinishing arditomason thebestofreno eeclongisland doktervanhecke famsales adla bogatylaw prefplastics kirbycontractinginc unlsp boban dottoressaromolimonica thebestofsanfrancisco vernix digitaldocuments tristatepropertybrokers sotrafib cemcorpny agenziaimmobiliarebuti joesitalianfoodmarket corpoguardiedicitta icsatc antoniniassicurazioni besel strappedincarseatsafety drevobeton blessingconstructionny centre-endorphine qmbtunisia elearning 357 mpress rrappliance ciaautomazioni antikva stubbanvel tacticalpublicrelations mpulshnk paulyboybrand lordshoes ijd-procom thebestrestaurants atkpalvelut ceat leonfukspc pilotexamssa ashgrovecabins universalshielding thebestofcharlotte johnjmazurinc rollnroaster thecabinetwarehouse pocketchangeduo thebestofmilwaukee resan sooli vwe centralwindowcleaning reinforcedplasticslab bugbustersofli ciandrigiardini arfada centrumeigenwijs thebestofmemphis maisonpearly islipll dcgraphicsinc agetranquille relisandroth thebestofcharleston traiteur-wn safi-ingenierie justmyvoice harrishardware louisbarbatolandscaping brightstartoursworld rga-insurance calacatajumper ezantia mrcheapocds van4holiday cigap richtour fagyhatar sicc weshopmall ventovuori sobatrapcapbon unityrubberllc thebestofsaltlakecity ircinc sescoindustries connply storen-servicesenter psnry greatneckcollision ironfitendurance tuscanycountryhouse sansovinocalcio bbradydesign samsam vdtarification springersoil thebestofdayton milantechnology labradoodlesoflongisland daliamohamed theconsultantpowerhouse gunnbrush kruunuosk shulmanproduce balcosupply conso-med federalnetworks bellmoreglass alwaysaffordableconcrete jayteeinsurance carolina-cabins cmorfinance stretchritepackaging dishaairwaysenterprise biocontrol distribio peterivill lcc inspekta jvidesigns royalroseinc calabriapizza fadhila prato-pronto-effetto arbemachine olsonelectricnj tkbl tuttifotokft alpernmd ggsupplywholesale almaxcorporation sotuflex ecustomgutter studenipotok metal-lineconcept rettsodontologi nutecsystems aclotbeach gjonnes-bygg cavalierinternational mnemos southfloridashuttles ajchemicalsupply rachelsfireisland concepto nesponge ultimatestylesofamerica techniquesmadeeasydrivingschool stevesmeatsfreeport eastwest gritbrush plussplan biosens viltkam wikaya paintballconcept justmyvoice securecarkeysupply justmyvoice allcountylegal catt plugandcharge ttandlcontracting justmyvoice islandboatlettering gms-tunisie sportsiena bloeiop touchofclasscollision kotekservice ittoscana pallongislandlacrosse industrialfinishings painoutband rakvag-batforening konemies rrfamilychiropractic infienile shbcgroup footpharmacydirect scuolaguidaprato ans-nettoyage autoskola saafa autolaky1 fixcars longislandelitelandscaping rayscan palaconstruction studio44 crealhome viniferi gavinburke ciprianigiardini davidpokorny osteriailcapodaglio thebestoffairfax centroorafofaccioli ateliervb bbdps thebestoffresno prodigus stratpak umisushirestaurant sotim suldalrenovasjon amer-equip k-kleven jerryspridepotatoes neuroky psicologozampoli jjslandscaping thebestofoklahomacity responsivesales paratie-antiallagamento-shop hearproof cfat ruspinameubles planetbioplastics lynbrook-plumber fcdf-ye brechanparkett valley-stream-plumber diagnosismaker aquavaria jedit garageennour heimdalbygg thebestoflittlerock raybomarine bayshorepaper gmstowing potatura-abbattimento-piante thebestoflouisville michaelalbert thebestofannarbor unitypavers mtnfueloil durub-mudiya lesgensdere dormerking thusney italianvistatravel maiemad gtiuniformcleaning suddenimpactli levituuli sourisalavie mayoiltank mschwartzfeather rands arieslimousines orthoticworld longislandcocktailhours waterjet allislandpaving dukediagnostic klfgoteborg yanezviaggi apruk decogato springeroilltd semapsolar irisgioiellicomprooro msedpsoftware fourcmanagement holemans 7consulting medinet evertile robertwitcomblandscape ceramics adrobotengineering volt-energy huisjacobs chimneyserviceboston eastmainstdental gcbt rememberingbriank elligiardiniespurghi paulslandscaping luisrestorations mgstunisie fixcarsny crcdd hotelprincipessalucca turvahallinta ralphjr thales irrigazione-giardini spantecsystems biomedic fgt-trading sols-egypt spectrumlaboratoriesinc prosecurite spongewarehouse afsainc dellafrancadevelopmentgroup leragazzedifirenze ferrettiwatches dovreentreprenor digitalhvac mostlymica fleurs-velghe autoscuolalebadie interlockingrubbertiles meadowcreekhoa bcn corpjetsupport jrkitchensflooring arteletti
Keywords
Angioedema, antiepileptic drug, carbamazepine, cutaneous reaction
Introduction
Carbamazepine an iminostilbene anticonvulsant drug commonly used for the treatment of neuralgia, seizure, and bipolar disorder. Cutaneous adverse reactions due to carbamazepine are reported to occur in about 3% of the population and mostly manifested as mild rash, erythema, petechiae, or exanthematous lesions.[1] Angioedema is an uncommon but serious hypersensitivity drug reaction often associated with angiotensin converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs, and penicillin therapy.[2] Prompt recognition and appropriate management of this complication are required to save the life. Although rare, literature review did show few cases of carbamazepine-induced angioedema.[1,3,4] We report here a rare case of carbamazepine therapy associated angioedema.
Case Report
A 34-year-old female, presented to the dermatology outpatient department of our tertiary care hospital, Puducherry, with generalized facial puffiness and itching all over the body. The patient was a known case of bipolar disorder and hypothyroidism and irregular treatment with tablet lithium 300 mg twice a day and tablet levothyroxine 150 μg once a day for the past 5 years. One-week before when the patient developed symptoms of agitation, anxiety, she was advised to continue lithium, levothyroxine regularly, and tablet carbamazepine 100 mg 3 times day was added to her treatment by a private practitioner. Twenty-four hours after the first dose of carbamazepine administration, the patient developed itching all over the body followed by generalized swelling of the face, periorbital area, and lips. The patient had no stridor or difficulty in breathing. The patient also stated that there was no history of allergic conditions, atopic dermatitis, or similar episodes in the past. She was treated with antihistamines (pheniramine maleate and hydroxyzine) and referred to our hospital for further management. On examination, diffuse swelling involving the face, periorbital area, and lips were noted. No other skin, mucocutaneous lesions or organomegaly was noted. Her pharynx was not erythematous and tonsils were normal. The chest X-ray findings were normal. Vitals were stable (pulse rate 82/min, respiratory rate 20/min and blood pressure 120/70 mmHg). Laboratory investigations showed normal values (total count of 7,900, SGOT 24 IU/L, SGPT 26 IU/L, blood urea 18 mg/dl, and serum creatinine of 0.7 mg/dl). Assessments of the complement system (CH50, C3, and C4) and serum carbamazepine level were not done. The offending drug carbamazepine was discontinued and the patient was treated with oral hydroxyzine 25 mg twice a day and topical emollients. Lithium and levothyroxine were continued and risperidone 8 mg once a day was added. The patient showed gradual but steady complete recovery with the treatment and was discharged after 5 days [Figure 1].
Discussion
Angioedema associated with the use of carbamazepine is a rare but potentially life-threatening reaction. Edema should be managed according to its clinical presentation. Angioedema can be categorized as hereditary or acquired with complications ranging from dysphagia to acute respiratory distress, airway obstruction, and death.[5] In our case, the symptoms were not severe, but developed only after carbamazepine administration and therefore it was possible to comprehend that the angioedema was induced by the same offending drug. The patient improved well with antihistamines without life-saving supportive treatment. However, the patient was not challenged with carbamazepine.
In this case, Naranjo’s algorithm was used to determine a plausible reaction due to carbamazepine.[6] The following criteria were considered: There were previous conclusion reports on this reaction (+1), the adverse event appeared after carbamazepine was administered (+2), adverse event improved when carbamazepine was discontinued (+1), adverse event reappeared when carbamazepine was re-administered (0), no alternate causes that could solely have caused the reaction (+2), the reaction reappeared when a placebo was given (0), drug detected in the blood (or their fluids) in a concentration known to be toxic (0), the reaction was more severe when the dose was increased or less severe when the dose was decreased (0), the patient had a similar reaction to carbamazepine in the previous exposure (0), and the adverse event confirmed by objective evidence (+1). Based on the total score of seven, the reaction was categorized as “probable” reaction to carbamazepine administration. According to the WHO-Uppsala Monitoring Centre causality assessment system, the cutaneous adverse reaction was found to be “probable/likely” reaction to carbamazepine.[7]
Angioedema is a vascular reaction involving deep dermis, subcutaneous or submucosal area characterized by asymmetrical nonpitting edema affecting any part of the body mostly confined to head and neck.[8] The pathophysiology is due to loss of integrity of blood vessels leading to plasma extravasation and consequently subcutaneous tissue edema mediated by the involvement of mast cells and in lesser extent basophils, which on activation release histamine from preformed granules. Prostaglandins, leukotrienes (LT) are known to be the other mediators involved.[3] It is postulated that drug-induced angioedema could be immunoglobulin E (IgE) mediated allergic reaction causing rupture of mast cells and histamine release. It may also due to nonimmunological mediated by interference with arachidonic acid metabolism resulting in LT over production (LTC4, LTD4, and LTE4). The other proposed mechanism is decreased degradation of bradykinin resulting in dilatation of blood vessel and inflammation.[5] In the absence of definite proof regarding the mechanism for the development of angioedema due carbamazepine, we can only speculate that it could be due to IgE-mediated allergic reaction.[3] Due to the potentially life-threatening complication of angioedema and frequent use of anticonvulsants in the treatment of mood disorders, treating physician must be aware of the condition and management. Patients should also be advised to report symptoms suggesting angioedema (swelling of the face, tongue, eyes, lips, or difficulty in breathing) immediately and to stop taking the drug until they have consulted their physician.
Conclusion
Angioedema is a rare but serious, life-threatening adverse reaction due to carbamazepine, which requires immediate treatment and monitoring by the treating physician in order to avoid further morbidity and mortality.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Mehta M, Shah J, Khakhkhar T, Shah R, Hemavathi KG. Anticonvulsant hypersensitivity syndrome associated with carbamazepine administration: Case series. J Pharmacol Pharmacother 2014;5:59-62.
- Kuriachan S, Amberkar MB, Mohan MK, Shahul HA, Kishore MK. Acebrophylline-induced angioedema. Indian J Pharmacol 2015;47:219-20.
- Elias A, Madhusoodanan S, Pudukkadan D, Antony JT. Angioedema and maculopapular eruptions associated with carbamazepine administration. CNS Spectr 2006;11:352-4.
- Gupta SN, Gupta VS. Angioedema caused by carbamazepine or acetazolamide: A single drug solution – An illustrative case report. Neurol Open J 2014;1:20-2.
- Inomata N. Recent advances in drug-induced angioedema. Allergol Int 2012;61:545-57.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
- The Use of the WHO-UMC System for Standardised Case Causality Assessment. Available from: http://www.who-umc.org/ Graphics/24734.pdf. [Last accessed on 2016 Mar 30].
- Lee A, Thomson J. Adverse Drug Reactions. 2nd ed. London: Pharmaceutical Press; 2006. p. 131-5.