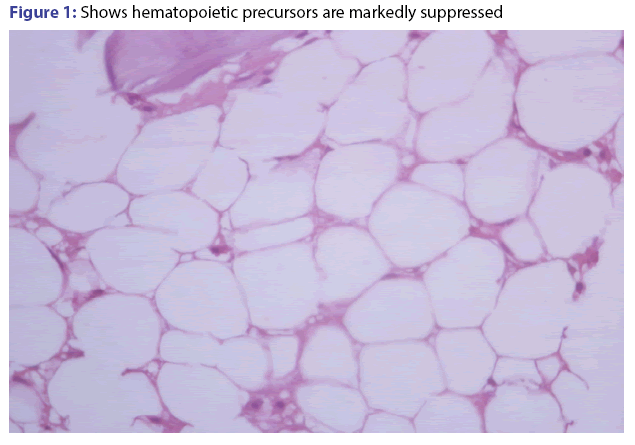
Capecitabine induced severe bone marrow aplasia
- *Corresponding Author:
- Dr. Irappa Madabhavi
Kerudi Cancer Hospital and Research Centre
Bagalkot, Karnataka, India.
E-mail: irappamadabhavi@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Here we are reporting rare case series of capecitabine induced marrow aplasia in patients of colorectal cancer during adjuvant treatment. First patient is 51 year old male with locally advanced rectal cancer, developed persistent pancytopenia after 5th cycle of chemotherapy. His bone marrow showed marked hypocellularity (3%). Second patient was 73 year old male with stage III adenocarcinoma of cecum & he developed asymptomatic bicytopenia. Bone marrow aspiration & trephine biopsy showed moderate hypo cellular marrow with bicytopenia. Third patient is 65 year old diabetic female with stage III periampullary adenocarcinoma. Initially started with CAPOX, but later in the 6th cycle of capecitabine, she developed pancytopenia. Bone marrow aspiration & biopsy showed pancytopenia with moderate to severe hypo cellular marrow. From the above three cases, it is evident that capecitabine causes hypocellular marrow, which we are using as adjuvant or palliative chemotherapy in colorectal cancer.
Key words
Capecitabine, marrow aplasia, colorectal cancer
Introduction
Capecitabine is an oral pro drug of 5-fluorouracil and used for the treatment of colorectal and other malignancies.[1] The common dose-limiting toxicities of capecitabine are hand-foot syndrome and diarrhea.[2]
Capecitabine induced transient neutropenia is seen but persistent pancytopenia due to severe hypo cellular marrow is not yet been reported. Capecitabine induced endothelial dysfunction, cytotoxic endothelial injury with sub endothelium exposure and thrombus formation has been observed in animal models. Increased endothelial thrombogenicity has been identified in animal models treated with 5-FU, indicating a toxic effect of 5-FU on endothelial cells.[3]
Case Vignette's
Case 1
Fifty-one-year-old male chronic smoker presented with symptoms of lower rectal mass, biopsy suggestive of mucinous adenocarcinoma and CT scan showed T4 lesion with perirectal lymph node involvement. His routine blood investigations were normal including complete blood counts (CBC).
Patient was treated with neo adjuvant concurrent chemo radiation with capecitabine. Complete R0 resection of rectal mass was done by low anterior resection. Post operative imaging, serum CEA, CBC was normal and planned for total 4 months of adjuvant CAPOX (capecitabine and oxaliplatin) for 6 cycles. No tolerance issue was present during first 2 cycles. 3rd and 4th cycles were delayed for 10 and 24 days due to persistent grade 3 neutropenia. Patient was given 3rd cycle with GCSF and prophylactic antibiotic support. 4th and 5th cycles were given with 20% dose reduction, GCSF and multivitamin support but all in vain.
Patient underwent marrow aspiration and biopsy after 5 cycle of CAPOX. It showed severe marrow aplasia (less than 10% cellularity) and all 3 cell lines were suppressed [Figure 1]. We gave him steroid trial for 1 month but count was not improved and was on observation for 3 months. Repeat marrow aspiration and biopsy showed less than 10% cellularity. Since 10 months after last CAPOX, his counts are persistently low. His last CBC was-HB 8.9/WBC 2300/cumm, platelets are 86000/cumm.
Case 2
Seventy-three-year-old male chronic smoker presented with ulceroproliferative growth in the cecum and biopsy suggestive of adenocarcinoma. His routine blood investigations were normal including complete hemogram. Patient underwent right hemicolectomy and post operative imaging, serum CEA, CBC was normal. In view of old age we started single agent capecitabine 1000 mg/m2 twice daily for 14 days with 1 week off as adjuvant chemotherapy for total 6 months. No tolerance issue was present during first 6 cycles. 7th cycle was delayed for 15 days due to persistent bicytopenia (WBC-2700/cumm and Platelets 75000/cumm) and was given at a dose of 850 mg/m2 PO twice a day on days 1-14 with GCSF and prophylactic antibiotic support. After the 7th cycle patient has persistent asymptomatic bicytopenia without any improvement with growth factor support and hematinics.
Patient underwent bone marrow aspiration and trephine biopsy suggested moderate hypo cellular marrow with bicytopenia. In view of old age we discontinued capecitabine and managed with hematinics only and he is asymptomatic, doing well without any complications.
Case 3
Third patient is 65-year-old diabetic female presented with weight loss, pain abdomen and loss of appetite since one month. CT scan showed circumferential thickening of the 2nd part of duodenum in the periampullary region. His routine blood investigations were normal including CBC. His CEA was 223 IU/ml. Patient underwent Whipple’s procedure and the specimen shows stage IIIA (T2N1a) duodenal adenocarcinoma. Post-operative imaging, serum CEA, CBC was normal.
She was planned for total 6 months of adjuvant CAPOX (capecitabine and oxaliplatin) chemotherapy treatment. After 2 cycles of CAPOX, patient developed grade 3 neuropathy, oxaliplatin was discontinued and continued with capecitabine only. No tolerance issue was present during first 5 cycles.
But later in the 6th cycle, she developed pancytopenia with Hb of 7.8 gm, WBC of 1800 and platelets of 49000. Patient was initially managed with growth factor support and hematinics but she didn’t get improved. Chemotherapy was delayed for 15 days. Bone marrow aspiration and biopsy was done which was showing pancytopenia with moderate to severe hypo cellular marrow. Eventually patient developed grade 4 febrile neutropenia on post chemotherapy day 10th of 6th cycle and managed with higher antibiotics in the ICU. After that we discontinued the chemotherapy and she is on monthly follow up with persistent pancytopenia, at our centre since 7 months.
All 3 patients underwent ELISA for Parvovirus B19 IgM and IgG antibodies, HIV, hepatitis B and hepatitis C, antinuclear antibody profile, direct Coombs test and all were negative.
Discussion
Capecitabine is inactive by itself and activated to 5-FU by 3 steps enzymatic conversion, then it exerts its cytotoxic action similar to 5-FU by 1) inhibiting thymidylate synthase and 2) its active metabolites which are incorporated into DNA and RNA.[1,4]
Capecitabine is a pro-drug of 5-FU, which is widely used in the treatment of several malignancies, i.e., colorectal, gastric, pancreatic, breast and head and neck cancer. Adverse hematologic effects are reported in less than 5% of patients receiving capecitabine as monotherapy, and include hemorrhage, coagulation disorder, idiopathic thrombocytopenic purpura, pancytopenia and bone marrow depression. Grades 3 and 4 neutropenia occurred with CAPOX in various randomized controlled trials.[5]
Capecitabine induced endothelial dysfunction, cytotoxic endothelial injury with sub endothelium exposure and thrombus formation has been observed in animal models. Most common explanations in the literature are 5-FU-induced vasospasm, direct vasoconstriction on smooth muscle cells (protein kinase C-dependent), increased endothelial thrombogenicity, indicating a toxic effect of 5-FU on endothelial cells. [3] But more studies are required to say conclusively involving robust number of patients and data. The molecular mechanism of 5-FU toxicity is deficiency of dihydropyrimidine dehydrogenase, leading to increased half life of 5-FU and therefore severe toxicity.[6]
Mortality rate following mono therapy or combination of treatment of capecitabine in CRC is 0.5-2%.[7] In a study of comparing CAPOX 2000 mg to CAPOX 1700 mg in 400 patients, did not detect any statistically or clinically significant differences in GI toxicities or hospitalization rates. But author concluded that there was reduced rate of neutropenia and liver dysfunction with the use of CAPOX 1700.[8]
Regarding the dose of capecitabine, 1 study encompassing 152 oncologists across India in metastatic breast cancer, is up to 1000 mg/ m2 twice a day.[9]
Conclusion
Capecitabine induced prolonged cytopenia is not a known entity and its exact mechanism is unclear. Capecitabine induced endothelial dysfunction, cytotoxic endothelial injury with sub endothelium exposure, thrombus formation, and 5-FU-induced vasospasm. From the above three cases, it suggests us that capecitabine is the culprit for marrow hypolpasia, but more studies are required to say conclusively involving robust number of patients and data.
Competing interests
None.
Acknowledgements
None.
References
- Miwa M, Ura M, Nishida M. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998;34:1274-81.
- Rosmarin D, Palles C, Pagnamenta A. A candidate gene study of capecitabine-related toxicity in CRC identifies new toxicity variants at DPYD and a putative role for ENOSF1rather than TYMS. Gut 2015;64:111-20.
- Panagiotis P, Justin S. Molecular Basis of 5-Fluorouracil-related Toxicity: Lessons from Clinical Practice. Anticancer Res 2014;34:1531-36.
- Noordhuis P, Holwerda U, Van Laar JA. A non-radioactive sensitive assay to measure 5-FU incorporation into DNA of solid tumors. Nucleosides Nucleotides Nucleic Acids 2004;23:1481-4.
- Cassidy J, Twelves C, Van Cutsem E. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin.Ann Oncol2002;13:566-75.
- Teng CJ, Hsieh YY, Chen KW.Sudden onset pancytopenia with intracranial hemorrhage after oxaliplatin treatment:a case report & literature review. Jpn J Clin Oncol 2011;41:125-9.
- Saltz LB, Niedzwiecki D, Hollis D. FOLFIRI is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer.JCO 2007;25:3456-61.
- Baird R, Biondo A, Chhaya V.Toxicity associated with capecitabine plus oxaliplatin in colorectal cancer before & after an institutional policy of capecitabine dose reduction. British Journal of Cancer 2011;104:43-50.
- Parikh PM, Gupta S, Parikh B. Management of primary and metastatic TNBC: Perceptions of oncologists from India. IJC 2011;48:158-64.