CACO-2 cell lines in drug discovery- An updated perspective
- *Corresponding Author:
Date of Received: 08-04-2010
Date of Modified: 25-04-2010
Date of Accepted: 02-05-2010
Available Online: 15-05-2010
Abstract
Cell lines are the invitro models used for the drug permeability studies in the preclinical and clinical phases of the drug discovery. Cell line models are simple and quick to use and avoids the usage of animal models for pharmacological and toxicological studies and hence cost effective, produce reliable and reproducible re-sults for understanding and evaluating the permeability characteristics of the potential lead drug candidates. Different cell line models used in the drug permeability studies, their characteristics has been summarized emphasizing on CACO-2. By virtue of its merits, CACO-2 cell line development, transport experiments, automated assays, opti-mization of experimental conditions and mechanistic uses of CACO-2 cell lines dealt comprehensively in the following context.
Keywords
Drug discovery, invitro cell lines, CACO-2, Drug premeability, Drug candidates
Introduction
evelopment of lead drug candidates (having potential pharmacological effect) derived from combinatorial synthesis has accelerated the drug discovery process. They have emerged as a quick and easy methods provides data on biological parameters like Potency, Absorption, Distribution, Metabolism, Excretion, Toxicity (ADMET) properties of large number of candidates can be determined simultaneously [1]. However limiting factors for the successful therapeutic application of new drugs is their ability to transport in biological systems namely, over the gastro intestinal tract (GIT), towards the target receptor, or across the blood brain barrier (BBB). Highly standardized invitro models are therefore required for screening of large number of molecules that gives predictive values for these transport processes. But however, absorption process is influenced by number of factors such as solubility, membrane partitioning, metabolism and transporters [2], [3], [4] . In contrast to less accurate experimentally based insilico predictions of intestinal permeability, done based on their molecular structure, behaviour of molecule with in a biological test system, mechanistic cell lines which gives better data is preferred. Therefore cell lines have evolved as a tool for understanding and evaluating the permeability characteristics of potential lead drug candidates.
Permeability Screening in Different Phases of Discovery
Screening of permeability of lead drugs in lead discovery phase (LD) results from High Throughput Screening (HTS) which provide data and basis for the further modifications of the structure of the molecule in lead optimization phase (LO) which ends with the selection of drug candidate (DC).Hence cell culture models provides a platform for preclinical screening, biopharmaceutical assessments & evaluation of mechanistic purposes [5].
Cell Cultures for Assessment of Intestinal Permeability
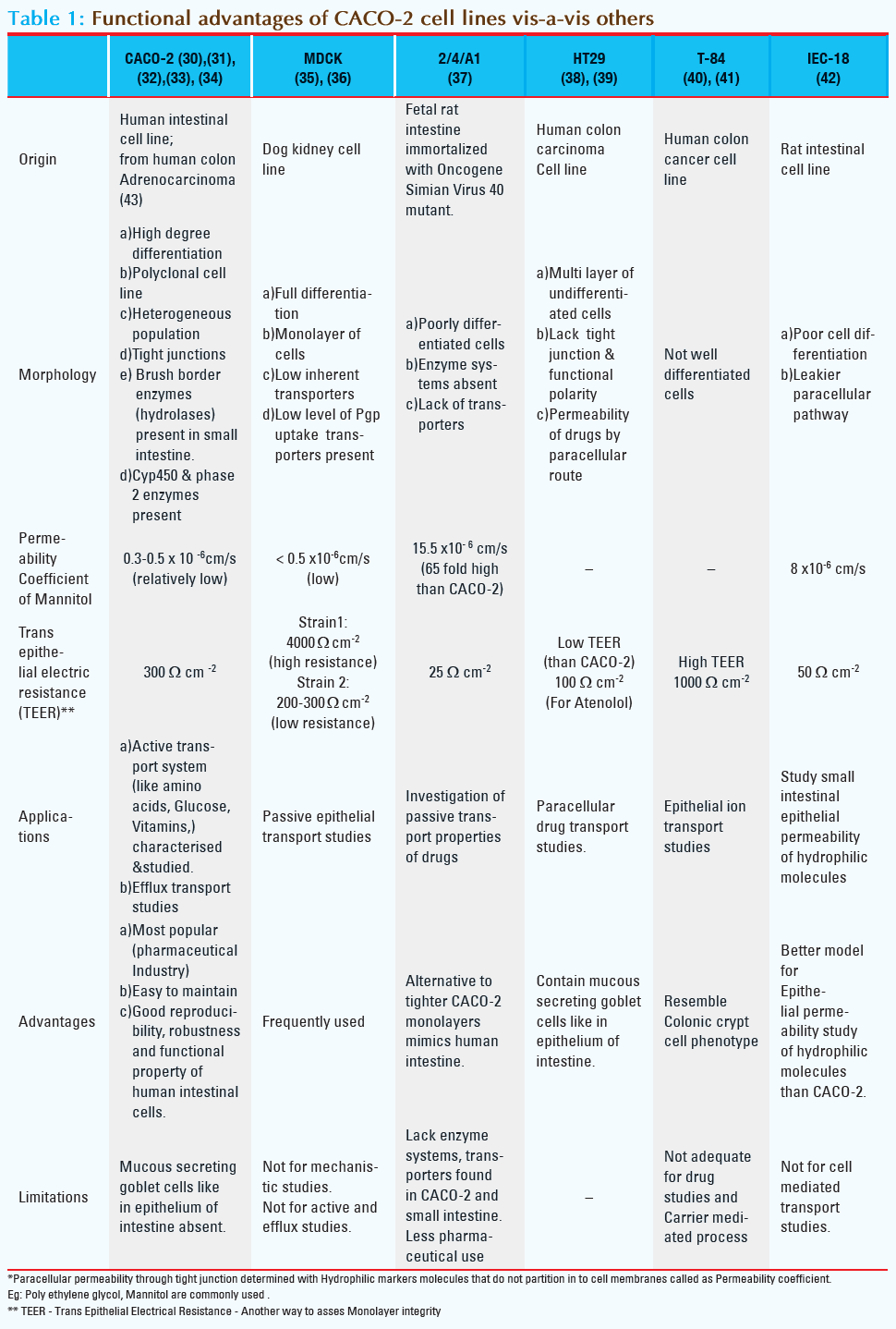
Cell culture based models are mainly used for studies of partitioning into membrane, mechanisms of drug absorption and interaction with epithelial proteins (such as transporters and enzymes).These are simple and quick to use, and they reflect different mechanisms involved in absorption process. Paracellular permeability is determined with hydrophobic marker molecules, that do not partition into cell membranes [6]. The functional characteristics of different cell lines currently used in drug discovery are summarized in Table-1.
CACO-2 Cell Line/Cell Culture and Transport Experiments
By virtue of the merits stated in the Table-1 CACO-2 cell line is most widely used to screen intestinal permeability, to know drug absorption, and to study passive and active diffusion transport of drug molecules.
Methodology [7], [8]
Cell lines used for screening are should be precultivated. The generalized culturing and experimental setup for CACO-2 cell model is as follows:
The cells are maintained on the filter inserts or culture flasks at 37°C in Dulbecco’s modified Eagle’s medium, 1% non-essential amino acid, 1.5% L-glutamine solution (200mM) and 9% fetal bovine serum and antibiotics under an atmosphere of 5% CO2.[Antibiotics- penicillin and streptomycin are used when cells grown on filter inserts]. Cultures are checked routinely for mycoplasma contamination. Cells are harvested from cell culture flasks by trypsinization - with a trypsin (0.25%) + EDTA (0.2%).
For transport studies CACO-2 cells are seeded on to specially designed culture inserts or filter inserts. Filter inserts of different membrane material and pore diameter are available. Some commercially available examples are Transwell inserts (Polysorbate; Corning Life sciences [9] and BD Falcon inserts (Polyethylene terepthalate (PET); BD Bioscience [10]. CACO-2 cells are directly grown on the filter inserts without extracellular collagen coating ,which is required for other cell lines for proper cell attachment and differentiation. Cell membrane inserts with narrow pore diameter (0.4μm) are generally used. Large pores results in monolayers growing on both sides of the membrane, resulting in depolarised cells and shall mislead the results. Seeding density should be between 0.5 and 5×105 cells/cm2. Cells are attached to the filters and are grown for 2 to 3 weeks [11], at this stage well differentiated monolayer intestinal epithelial cells having morphological and biochemical properties that resemble those of normal intestinal (absorptive) enterocytes are formed.
In drug absorption studies, drug is added to apical (mucosal site) and drug appearance on the basolateral side (serosal) is studied by time .These model also permit experiments carried out in reverse direction (basolateral to apical). Drug absorption experiments are performed at 37°C under sink conditions in order to avoid back diffusion. Trans epithelial electrical resistance (TEER) should be measured prior to and after experiment to verify monolayer integrity.
Apparent permeability coefficient (Papp) is calculated by equation:
Papp = (dq/dt)/(A x Cdo)
dq/dt - rate of appearance of drug on the receiver side,
Cdo - initial drug concentration on the donor side,
A - surface area of the filter membrane.
This equation is improved by taking in to account the change of donar concentration (Cd),which affects driving force for passive diffusion and concentration gradient.
Thus the modified equation Papp = k x Vr/A.
k - change in drug concentration in the receiver chamber (Cr-ti/Cd-t) per unit time,
Cr-ti - concentration on the receiver side at the end of each time interval,
Cd-ti - average of the donor concentration determined at the beginning and at the end of each time interval,
Vr - volume of the receiver chamber, and
A - surface area of the filter membrane.
Automated CACO-2 Assay
Automated CACO-2 permeability assay is performed using semi or fully automated procedures. Using this system it is possible to obtain a throughput of approximately 400–500 compounds per week. Automated CACO-2 assay systems are commercially available through Tecan [12], BD Bioscience and Bohdan, Mettler Toledo [13]. In addition, automated systems for maintenance of cell cultures are commercially available, while totally automated systems for both maintenance and culturing of cells grown on permeable filter supports are under development by companies such as The Automation Partnership [14].
Solubility of test compounds measured to make correct determination of the permeability coefficient.Test compounds are first prepared in dimethyl sulfoxide (DMSO) solution (1mM) on parent plate and diluted in transport buffer to give final drug concentration (10μM) and is added to the cell monolayers. In automated assay, incubation, shaking, pippeting performed by a robotic microplate processor. Preparation of drug donor solution and standard carried out on multiple probe, 14 to 18 day old CACO-2 monolayers on polycarbonate filters are used fot this purpose and TEER also measured. The assay is developed to handle 4 × 24 (96) well plates with monolayers. Duplicate transport measurements for each substance and mixture of three reference standards are included to capture variabilities of assay that arise by time or passage across the cell line.
In the robotic systems the basolateral chamber is filled with transport buffer, after which donor drug solution is added to apical side of CACO-2 monolayer. Samples are taken immediately from the donor side to determine the initial donor concentration. The appearance of compound on the receiver is measured by taking samples from the receiver side at 45 and 120 min. At the end of the experiment, samples are also taken from the donor side in order to measure the change in donor concentration and calculate mass balance (recovery) of the transport process. In general, the samples are collected in deep well 96-well plates, diluted in acetonitrile and then analyzed for drug content by LC/MS. The recovery should be kept within limited ranges in order to assure highquality and accurate Papp values. Limits for recovery are between 80 and 120%.
Recovery % is calculated by
Recovery (%) = (Mr-120 + Md-120/Md-o) × 100
Mr-120 - cumulative amount of drug transported to the receiver side at the end of the experiment (including amount removed in sampling), Md-120 - amount of drug remaining on the donor side at the end of the experiment, and Md-0 - amount of drug on the donor side at the start of the experiment (determined from the initial donor concentration sample).
Quality Control and Standardization of CACO-2 Assay
For reproducible and high-quality results, a routine characterization should be performed on each new batch of cell monolayers by measuring TEER and permeability to a paracellular marker such as 14C-mannitol.The morphology and integrity of the monolayers should be examined using Transmission Electron Microscopy (TEM) and Fluorescence microscopy.
The following parameters should be standardised [15]
• Filter supports and growth media.
• C ulturing protocols, seeding density and trypsinization procedures.
• Microscopic verification of monolayer morphology.
• Reproducibility of the relationship between f raction absorbed Fa (%) and Papp for a defined set of reference drugs.
• I nternal standards or markers and acceptance criteria
• Specified experimental conditions (e.g., pH, stirring, drug concentration, Papp calculation).
• C haracterization of expression and function of important enzyme and transport systems.
These suggested factors for standardization of the CACO-2 model might also be reasons for the variability in published permeability values obtained from different laboratories. The CACO-2 cells are used at various passage numbers between 20 and 110. CACO-2 cells obtained from American type culture collection (ATCC) and European collection of cell cultures (ECACC) are usually at passage number 20–40. Cells of different passage number can have very different properties with respect to paracellular permeability and functional expression of transporters. It is recommended that the cells are used within a relatively limited range of passages for which the cells properties have been well characterized.
Optimization of Experimental Conditions [16], [17]
a) pH
The pH in the gastro intestinal tract (GIT) is a very important factor affecting drug absorption for ionisable drugs. pH has two main effects on drugs: one is to determine the degree of ionization (dissociation) of weak electrolytes and thereby affect the passive diffusion of the compound; and the other is to establish a proton gradient as a driving force for some transporters.
The pH in the lumen of the human GIT is variable; typically it is pH 1–2 in the stomach, 5–6.5 in the duodenum and proximal jejunum, 6.5–7.5 in the mid jejunum, and almost up to 8 in the terminal ileum. In the large bowel the pH varies between 6.5 and 8 from the colon ascendens to the sigmoideum. This bulk pH will affect mainly the solubility of the compound and is also thought to effect the ionization that provides a driving force for the regional difference in uptake of mainly the uncharged form. Actual transport across the epithelial membrane is affected by another pH, the so called microclimate or surface pH(pH=5.5) in the upper small intestine.
The pH of the apical solution has a direct impact on the transport experiments using monolayers, since the solution is in direct contact with the membrane and should therefore mimic the microclimate pH. The pH on the basolateral side should in all cases refl ect the pH of the extracellular fluid within the submucosa, which is under direct influence of the pH in the blood. Suggested examples for quick evaluation of the pH effect (apical/basolateral) on transport in different regions of the intestine include:
• For jejunal transport = 6.0/7.4
• For ileal transport = 7.4/7.4
• For colon transport = 7.4/7.4
For weak acids, e.g., salicylic acid, the dependency on a pH gradient becomes complex since both the passive diffusion and the active transport process will be dependent on the proton concentration in the apical solution and a lowering of the pH from 7.4 to 6.5 will increase the apical to basolateral transport more than 20-fold. Similarly, for weak bases such as alfentanil or cimetidine, a lowering of the pH to 6.5 will decrease the passive transport towards the basolateral side. The transport of the ionisable compound will, due to the pH partition hypothesis, follow the pKa curve. In an early screening situation when the transport process is unknown, it can be difficult to distinguish a passive asymmetric uptake or efflux due to the pH effect on ionization from a true carriermediated uptake or efflux. The following assays are therefore suggested:
• If screening for general intestinal absorption is equal to pH gradient system, only report to projects Apical to Basolateral direction
• If screening for carrier-mediated transport in any direction is equal to both a nongradient and a pH gradient system should be used and bidirectional transport data should be reported.
b) Solubility and Bovine Serum Albumin (BSA)
Solubility data are vital in order to improve the quality from the biological assays. Optimizing the permeability measurements to avoid adsorption to plastic, filters or accumulation within the cell monolayer seems highly relevant for increasing the predictivity of the screening model in early screening in the determination of permeability values. To perform high-quality permeability measurements, using a cell culture-based model, a limit for low solubility has to be drawn. If the drug is not soluble within the experimental system then the data will be of low quality and affect the recovery estimates from the permeability assay. The solubility limit to be set is dependent upon several factors, e.g., analytical, enzymatic and whether (or not) transporters are involved. Therefore, a balance between solubility, analytical and permeability issues must be considered before conducting a large number of assays with lipophilic drugs.
The effect of the presence of BSA will be determined by both the protein binding capacity of the drug to be tested and its intrinsic permeability, i.e., high protein binding and high permeability values will increase the response to the presence of BSA in the basolateral chamber BSA used to increase sink conditions and to reduce the adsorption phenomenon. However, there seems to be a concentrationdependent effect of BSA, which has been tested between 0 to 4 %.
Mechanistic Use of Cell Models
For the evaluation of a possible relationship between the molecular structure of a potential candidate and its transport abilities to cross the epithelial membrane of the gut, the mechanism or route of transport must be known. During the lead optimization phase – when many mechanistically based studies are performed – the cell culture-based models can also be used with great confidence [18].
Paracellular Pathway [19]
Compounds are not efficiently absorbed due to the small available absorptive area and the restriction by tight junctions. CACO-2 cells possess lower permeability towards polar molecules than does the small intestine invivo because the monolayer has a much smaller effective surface area than the small intestine villi, due to the tight junctional resistance [20] . The paracellular pathway, between the epithelial cells, is both size- (molecular weight, volume) and chargedependent. The molecular weight cut-off seems to be around 300 g mol-1 for the CACO-2 cell monolayer, which shows the more colonic nature of the CACO-2 monolayer model.
Transcellular Pathway [20]
Molecules with a large molecular weight or size are confined to the transcellular route and its requirements related to the hydrophobicity of the molecule. The transcellular pathway has been evaluated for many years and is thought to be the main route of absorption of many drugs, both with respect to carriermediated transport and passive diffusion. Molecules with a large molecular weight or size are confined to the transcellular route, transcellular pathway suggested for proteins and peptides is the transcytotic pathway, which involves a receptor-mediated endocytosis in CACO-2 cells.
Carrier-Mediated Transport [21], [22], [23]
Transporters like aminoacid and oligopeptide-carriers (peptide transporters PepT1 and PepT2) are involved in the absorption process of pharmaceutical drugs. The organic anion transporters (OAT) and PepT1 is expressed in the cell lines of CACO-2 and HT-29.Cell lines, such as the CACO-2 and MDCK cells have been used frequently to study different transporters in the GI tract. These cell lines have been evaluated for transport both in absorptive and secretory direction, and in addition also been transfected with specified transporter systems of interests to yield new clones or co-cultures.
Application of Cell Monolayers and Integrity Measurements
I. Evaluation of Metabolism During Transport [25]
• Cell culture models can be used to evaluate the importance of metabolism in gut membranes, both with respect to oxidative metabolism via the Cytochrome P450system and Phase-2 reactions
• CACO-2 model can in fact serve as an excellent model to investigate both permeation and first-pass metabolism.
II. Evaluate the toxicity of compounds;
III. Evaluate the toxicity of excipients in formulations;
IV. Establish test solutions for optimal screening; and
V. Evaluate formulations containing enhancers of intestinal permeability [26].
Computational Models for Prediction of Intestinal Permeability [27]
• Reduce the time and cost to select potential candidates as well as to complement the biological screening and optimization processes because there is no need to synthesize compounds.
• Several commercially available tools/software using CACO-2 cells as a model for intestinal permeability have also been established, e.g., VolSurf (Tripos Inc.) [28] and LION’s iDEA (LION Bioscience, Inc.) [29]
Conclusion
Cell lines are well suited as high capacity screening models, biopharmaceutical assessments (like ADMET, potency properties), and for mechanistic evaluation of transport mechanisms useful in pre clinical and clinical phases of the drug discovery. Cell lines with emphasizing on the CACO-2 models form cost effective, less time consuming, quick and easy invitro methods generates high throughout, reliable and reproducible data. Several computational and mechanistic models are commercially available for prediction of intestinal permeability by which, one can predict absorption of drugs in human GIT.
References
- Zam ora I, Oprea T I, Ungell AL. Prediction of oral drug permeability, in: Rational Approaches to Drug Design. Holtje, H.D., Sippl, W.(eds), Prous Science Press, Barcelona,2001:271–280.
- Ungell, AL, Invitro absorption studies and their relevance to absorption from the GI tract, Drug Dev. Indust. Pharmacy1997; 23:879–892.
- Ungell AL, Abrahamsson B. Biopharmaceutical support in candidate drug selection, in: Pharmaceutical Preformulation and Formulation .A Practical Guide from Candidate Drug Selection to Commercial Dosage Formulation. Gibson, M. (ed.),Inter pharm Press, 2001.
- Balimane PV, Chong S,Morrison RA. Current methodologies used for evaluation of intestinal permeability and absorption J. Pharmacol.Toxicol. Methods 2000; 44:301–312.
- Stevenson C L, Augustijns P F, Hendren R W. Use of Caco-2 cells and LC/MS/MS to screen a peptide combinatorial library for permeable structures, Int. J. Pharm.1999; 177:103–115.
- Taylor EW, Gibbons JA, Braeckman RA .Intestinal absorption screening of mixture from combinatorial libraries in the Caco-2 model, Pharm. Res. 1997; 14: 572–577.
- Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport, Adv. Drug Deliv. Rev.1996; 22:67–84.
- Wilson G, Hassan IF, Dix CJ, et al. Transport and permeability properties of human Caco-2 cells: an in vitro model of the intestinal epithelial cell barrier, J. Controlled Release 1990; 11: 25–40
- http://catalog2.corning.com/Lifesciences/enUS/Shopping/ category.aspx?categoryname=Cell%20Culture%20and%20 Bioprocess(Lifesciences)
- http://www.bd.com/india/products.asp
- Tavelin S, Milovic V, Ocklind G, et al. Conditionally immortalized epithelial cell line for studies of intestinal drug transport, J.Pharmacol.Exp. Ther.1999; 290:1212–1221.
- http://www.biodirectusa.com/tecan_freedom_evo_rsp_safire2_ genios.php
- http://in.mt.com/in/en/home/products/L1_AutochemProducts. html?als=autochem
- http://www.automationpartnership.com/tap/custom_automation/ index.htm
- Yazdanian M, Glynn, Hawi H, et al . Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds, Pharm.Res. 1997; 15: 1490–1494.
- Yamashita S, Furubayashi T, Kataoka, et al. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells, Eur. J.Pharm. Sci. 2000; 10: 195–204.
- Palm K, Luthman K, Ros J, et al. Effect of molecular charge on intestinal epithelial drug transport: pH dependent transport of cationic drugs. J. Pharmacol.Exp.Ther.1999; 291: 435–443.
- Artursson P, Ungell AL, Lofroth JE, Selective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments, Pharm. Res. 1993; 10: 1123–1129.
- TanakaY, TakiY, Sakane T, et al. Characterization of drug transport through tight-junctional pathway in Caco-2 monolayer: comparison with References 123 isolated rat jejunum and colon, Pharm. Res. 1995;12: 523–528.
- Yamashita S, Tanaka Y, Endoh Y, et al. Analysis of drug permeation across Caco-2 monolayer : implications for predicting in vivo drug absorption, Pharm. Res. 1997;14: 486–491.
- Sai Y, Kajita M, Tamai I, et al. Adsorptive-mediated transcytosis of a basic peptide, 001-C8 in Caco-2 cells, Pharm. Res. 1998;15: 1305–1309.
- Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs, Pharm.Res.1996; 13: 963–977.
- Guo A, Hu P, Balimane, et al. Interactions of a nonpeptidic drug, valacyclovir, with the human intestinal peptide transporter (hPePT1) expressed in a mammalian cell line, J. Pharmacol. Exp. Ther.1999; 289: 448–454.
- Wenzel U, Gebert I, Weintraut H, Weber WM, et al. Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PepT1 and in human intestinal Caco-2 cells, J. Pharmacol. Exp. Ther.1996; 277: 831–839.
- Gan L, Thakker DR. Applications of the Caco-2 model in the design and development of orally active drugs: elucidation of biochemical and physical barriers posed by the intestinal epithelium, Adv. Drug Deliv. Rev. 1997; 23: 77–98.
- Anderberg EK, Artursson P. Cell cultures to access drug absorption enhancement, in: Drug Absorption Enhancement. de Boer. A. G. (ed.), Harwood Academic Publishers, 1994: 101–118.
- Egan WJ, Lauri G.Prediction of intestinal permeability, Adv.Drug. Del. Rev. 2002; 54:273–289.
- http://tripos.com/index.php?family=modules,SimplePage,syby l_volsurf
- http://www.lionbioscience.com/
- Hidalgo IJ, Li J. Carrier-mediated transport and efflux mechanisms in Caco-2 cells, Adv. Drug Delivery Rev.1996; 22: 53–66.
- Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model for intestinal epithelial permeability, Gastroenterology1989; 96: 736–749.
- Cell Cultures in Drug Discovery: An Industrial Perspective Walter E, Kisse T. Heterogeneity in the human intestinal cell line Caco-2 leads to differences in transepithelial transport, Eur. J.Pharm. Sci.1995; 3: 215– 230.
- Delie F, Rubas WA. Human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model, Crit. Rev. Ther. Drug Carrier Syst.1997; 14: 221–286.
- Hidalgo I J, Assessing the absorption of new pharmaceuticals, Curr. Top.Med. Chem.2001; 1: 385–401.
- Irvine JD, Takahashi L, Lockhart K, et al .MDCK (Madin-Darby Canine Kidney)cells: a tool for membrane permeability screening, J. Pharm. Sci. 1999;88:28–33.
- Cho MJ, Thompson DP, Cramer, et al.The Madin Darby canine kidney (MDCK) epithelial cell monolayer as a model cellular transport barrier, Pharm. Res. 1989; 6: 71–77.
- Tavelin S, Milovic V, Ocklind G, et al. Conditionally immortalized epithelialcell line for studies of intestinal drug transport, J. Pharmacol. Exp. Ther.1999; 290: 1212–1221.
- Wikman A, Karlsson J, Carlstedt I ,et al. A drug absorption model based on the mucus layer producing human intestinal goblet cellline HT29-H, Pharm. Res. 1993;10: 843–852.
- Wikman-Larhed A, Arursson P. Co-cultures of human intestinal goblet(HT29-H) and absorptive (Caco-2)cells for studies of drug and peptide absorption, Eur. J. Pharm. Sci. 1995;3: 171–183.
- Brayden DJ. Human intestinal epithelial cell monolayers as prescreens for oral drug delivery, Pharm. News 1997; 4:11–15.
- Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells, Biochem. Biophys. Res. Commun 1991;175: 880–885.
- Duizer E, Penninks AH, Stenhuis WH, et al. Comparison of permeability characteristics of the human colonic Caco-2 and rat small intestinal IEC-18 cell lines, J. Controlled Release 1997;49: 39–49.
- Fogh J, Fogh J M, Orfeo T. One hundred and twenty-seven cultured human cell lines producing tumors in nude mice, J. Natl. Cancer Inst. 1977;59: p.221–225.