Branded versus Generic (Branded-Generic) Medicines-For Whose Benefit?
- *Corresponding Author:
- Sonali P Suryawanshi
Assistant Professor, Department of Pharmacology, Bharati Vidyapeeth Deemed University Medical College, Katraj, Dhankawadi, Pune-411 043, Maharashtra, India.
E-mail: docssurya@gmail.com
Citation: Suryawanshi SP, Totlani PS, Sahasrabudhe RA. Branded Versus Generic (Branded-Generic) Medicines-For Whose Benefit?. J Basic Clin Pharm 2017;8:158-161.
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Introduction: Cost of medicines is a major concern today. Generic drugs provide major saving opportunity in healthcare expenditure since they usually have lower price. However, physicians and patients are apprehensive regarding their quality, although they are bio-equivalents of the innovator products. The present study therefore compares the price structure and antibacterial activity of the branded products and their branded-generic counterparts. Methodology: Commonly used branded and branded-generic versions of three oral cephalosporins available in the pharmacy were studied. Health cost was compared by comparing Maximum retail price (MRP) and price-to-retailers (PTR) and trade margins. The antibacterial activity of the pairs was assessed by “Kirby Bauer” disk diffusion method, by comparing the zones of inhibition. Results: The retailer margin for the three branded medicines was in the range of 17-25% but for their branded-generic versions, it was huge, in the range of 73-130%. By switching over to branded generic medicines cost benefit to pharmacist ranged from 270% to 422% but for patients it was only 5%-48.3%. There was no difference in the zones of inhibition obtained for the branded generic and the corresponding branded drugs, indicating equal effectiveness. Conclusion: Branded generic cephalosporins, equi-effective as the branded versions, with lesser cost to the patient, can be used clinically. However, cost benefit to the patients, being hyped in the media, is very meager compared to the profit margins for the retailers. Steps are needed to ensure that the cost benefit with the use of generics reaches the patients in appropriate amounts. mp3download.link Best YouTube to MP3 converter. Download MP3 from YouTube for Free. one would honestly have expected more on the track https://www.mp3-go.net Download Mp3 songs for free Given the legendary pedigree of the man behind the sound downloadmp3-gratis.biz Download mp3 songs online at Mp3 Converter, watch high quality online music videos download-mp3gratis.me watch and download free songs of the highest quality. Listen to songs online here comfortably without any annoying advertisements. metrolagu.site Easy to use and free MP3 downloader. YouTube To MP3 download in seconds using the best YouTube to MP3 converter. YouTube To Mp3 Get the latest song by simply typing the latest artist or song title in the Search menu. Mp3 file format with 128 - 320 Kbps bitrate converted from YouTube videos. read at this blog All those artist performances are still available on YouTube today find more here
Keywords
Branded-branded generics, cost of medicines, antimicrobial activity
Introduction
India is the 4th largest producer and 10th largest exporter of drugs in the world. In spite of Government provision for health care budget, major proportion Indian population does not have access to even essential medicines.[1] With the sky-rocketing healthcare costs, the interest in generic drugs has increased all over the world, amongst rich or poor. Generic drugs enable major savings in healthcare expenditure since they are usually substantially lower in price than the innovator brands. [2] In India, in view of poor accessibility and affordability of people, it is absolutely essential that the generic drugs should be made available to minimize the cost of treatment. Reported production and use of generic drugs had jumped from 49% of the global drug market in 2000 to 78% in 2010.[3] In India, many pharmaceutical companies manufacture a product under both types, i.e., the branded product which they advertise and push through doctors and branded-generics which they expect to be sold over the counter by retailers. Generic drugs in simple terms are the copy of the branded ones having same ingredients, same dosage, same indications and exactly same pharmacological effects, as the manufacturing companies use the same active ingredients in both type of formulations. However, physicians are apprehensive regarding the quality of generic drugs [4] Consumer awareness for the generics, variety of trade names available in the market, and price variation is also very limited. Though in certain parts of India, government authorities recommend prescribing drugs by their generic names, drugs continue to be prescribed by the brand names and Doctors as well as patients do not want pharmacists to change the trade name written by doctor, despite the possible cost benefit. The present study was therefore undertaken to compare the price structure and antibacterial activity of the branded products and their branded-generic counterparts after the institutional ethical clearance. The same study had also been selected for ICMR–STS 2016-17.
Objectives
1. To compare the Price-to-patient (MRP - Maximum Retail Price) and Price-to retailers (PTR) and Trade margin of “branded” and “branded-generic” equivalents of some commonly used oral cephalosporins available in the tertiary care hospital pharmacy.
2. To compare the antibacterial activity of these formulations of cephalosporins.
Methodology
Three commonly used oral cephalosporins available as branded and branded-generic versions were selected. Basic information of the brands used was recorded from the formulation packs [Table 1].
| Cephalosporin | Code | Batch No | Mfg date | Expiry Date |
Brand Name & Manufacturer |
|---|---|---|---|---|---|
| Cephalexin | B1 | 2706924 | 6/2015 | 2/2017 | Sporidex 250 Sun pharma |
| BG1 | B651155 | 11/2015 | 6/2017 | Cephadex 250 Cipla |
|
| Cefuroxime Axetil | B2 | 5133176 | 12/2015 | 11/2017 | Zocef 500 Alkem |
| BG2 | WBT-5240B | 10/2015 | 9/2017 | Bullcef 500 Ultra-Drugs Pvt. Ltd. |
|
| Cefixime | B3 | EI 51546 | 11/2015 | 10/2017 | Ziprax-200 DT Cipla |
| BG3 | TX-7718 | 05/2015 | 4/2017 | Cefixar-200 DT Legen healthcare |
B1, B2, B3 - Branded products, BG1, BG2, BG3-Branded Generic products.
Table 1: Details of the drug products selected for comparative analysis.
Difference in cost
Medicines are available to patients at the MRP mentioned on the package of medicine. PTR is the price at which wholesaler (distributor) sells the product to the retailer and was noted from the purchase rate vouchers. Price-to-patient and price-to-retailers was analyzed for all the “pairs” of cephalosporins and Trade Margin for the retailer was calculated by using the formula:
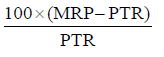
We also calculated the percentage increase in trade margin for the retailer and % cost benefit to the patient on switching to generic products.
Antibacterial activity
The antibacterial activity of all the selected formulations of the 3 cephalosporins against Escherichia coli and Staph aureus species isolated from different clinical samples was tested by “Kirby Bauer” disk diffusion method according to the ‘Clinical and Laboratory Standards Institute’ (CLSI) guidelines.[5]
The present study was performed with 30 μg Cephalosporin containing discs. The 3 Cephalosporins were coded as 1, 2, 3 with suffix B for branded and BG for branded generic drug in each pair. The 30 μg Cephalosporin discs of different brands were prepared by serial dilution using phosphate buffer 6 or 7 for the available strength of tablets or capsules. The discs were soaked for 24 hours and refrigerated. Antibacterial activity was tested the next day. Zones of inhibition was measured in mm after 24 hours of incubation by CLSI / Eucast reference for Disk diffusion (Kirby-Bauer) method.[6,7]
Data analysis
Cost aspect was analyzed by comparing the PTR, MRP, Trade margin and percentage difference in trade margin for the retailer and cost benefit to the patient on switching to the generic brand. For measurement of antibacterial activity, zone of inhibition was measured in millimeter (mm).
Observations and results
The samples of the 6 formulations obtained were stored according to the manufacturer’s packaging instructions and kept there until testing. The microbiologist who conducted the study was kept blinded. The description of the codes for all the brands and the details of drug products selected for this study are given in Tables 1 and 2.
| Pair Sr.No |
Drug Name | PTR | MRP | Trade Margin % |
On switching to branded generics | |||
|---|---|---|---|---|---|---|---|---|
| increase in trade margin | % increase in trade margin | Cost benefit to the patient | % Cost benefit to Patient | |||||
| 1 | Cephalexin (B1 | 84.8 | 106 | 25 | 105.5 | 422% | 45.2 | 42.6% |
| Cephalexin (BG1) | 26.40 | 60.8 | 130.5 | |||||
| 2 | Cefuroxime Axetil (B2) | 460 | 557 | 21.08 | 58.92 | 270% | 269 | 48.3% |
| Cefuroxime Axetil (BG2) | 160 | 288 | 80 | |||||
| 3 | Cefixime DT (B3) | 81 | 95 | 17.28 | 55.79 | 322% | 5 | 5% |
| Cefixime DT (BG3) | 52 | 90 | 73.07 | |||||
PTR - Price to the retailer; MRP- Maximum retail price; Branded Generics/Branded drug products and their trade margins
Table 2: Cost benefit to pharmacist and patient.
The above table shows the basic details of the procured branded and branded generic cephalosporins. All the drugs studied during May- June 2016 were manufactured in 2015 and had expiry in 2017.
Assessment of cost benefit
Table 2 shows the cost benefit to pharmacist (Trade margin) and patients with the use of selected branded generic drugs. It is seen that the trade margin which is 25% with branded cephalexin increased to 130.5% with branded generic cephalexin–effectively increasing the profit to the pharmacist by 422%. With the difference in the MRPs of the two products, cost benefit to the patients is 42.6% which is just 10% of the benefit to the retailer Thus, the study reveals that there are huge mark-ups for retailers on branded-generic medicines because of significantly lower PTR. The retailer margin for three branded medicines studied was in the range of 17-25%, but for their branded-generics version, it was in the range of 73-130% [Table 2]. By switching over to branded generic medicines cost benefit to pharmacist increased from 270% to 422%. Patient benefit depends on the difference in MRP. For the three drugs studied, patient benefit was only 5%-48.3%.
Assessment of antibacterial activity
We have compared the antibacterial activity of branded and branded generic formulations of 3 oral cephalosporins using disk diffusion method (Kirby-Bauer method). Each tablet in the respective pair had the same strength [Table 1]. The results of the study in terms of diameters of zones of inhibition produced by the different brands of cephalosporin tablets against the tested bacterial strains are given is given in Table 3. Photographs of the plates with the zones of inhibition are given in Figure 1. According to Table 3, amongst the three pairs of oral cephalosporins, the zones of inhibition did not differ between the branded and branded generic formulations. In fact, the zones of inhibition for the branded generics were slightly larger than those for the corresponding branded formulations.
| Pair Sr. No. |
Drug Name | Zone of Inhibition (mm) | Recommended Zones of inhibition (mm) | ||
|---|---|---|---|---|---|
| E. coliATCC | Staph ATCC | E. coli | S. aureus | ||
| 1 | Cephalexin (B1) | 18 mm | 25 mm | 15-21 | 29-37 |
| Cephalexin (BG1) | 21mm | 33mm | |||
| 2 | Cefuroxime Axetil (B2) | 15mm | 15mm | 20-26 | 27-34 |
| Cefuroxime Axetil (BG2) | 20mm | 15mm | |||
| 3 | Cefixime DT (B3) | 20mm | 10mm | 23-27 | |
| Cefixime DT (BG3) | 21mm | 15mm | |||
Table 3: Zones of Inhibition of different brands of oral cephalosporin.
Discussion
The Original concept of Generics distinguishes these products form the ‘patented’ or ‘innovator’‘ products marketed by a company who has invested a lot of time, money and effort on the research. Once out of patent, the drug can be manufactured by other companies who have not invested in it at the research level and hence their brands can be available at a lesser cost to the patients. In low and middle income countries, originator brand medicines generally cost substantially more than their generic equivalents. Generic drugs provide the opportunity for major savings in healthcare expenditure.[2] In India the concept has a completely different connotation. Branded does not mean innovator patented products, but brands on which the company spends a lot for marketing, against the generics which are sold by giving huge incentive to the retailers.
Generics are supposed to be marketed and prescribed by the generic name of the drug. However, physicians are apprehensive regarding the quality of generic drugs.[4] That has led to the emergence of the so called ‘branded generics’, wherein the same reputed companies started manufacturing a product under both types, i.e., the branded product which they advertise and push through doctors and branded-generics which they expect to be pushed over the counter by retailers.
This is one of the first studies in India conducted systematically to compare difference in health care cost and antimicrobial efficacy of three oral cephalosporins available as branded and branded generic products. Efforts were made to procure these products having close manufacturing and expiry dates [Table 1].
Findings of the study revealed that though these branded generics are available to the patients at a somewhat lower cost than the corresponding branded formulations, this cost benefit is negligible as compared to the tremendously higher trade margins they offer to the retailers, who therefore willingly promote the sale of such (branded generic) products.
Other studies in India comparing different generic medicines to their branded counterparts also show that price-to-patient for the branded-generic version was not much less than to its branded counterpart; the price difference being only 71-100% of the branded formulation.[8,9]
Since there is not a substantial difference in the MRPs of these products, this higher trade margin is possible because of the very low PTR for the branded generics. PTR reflects the manufacturing cost of the product. This naturally leads to a question about the quality of generic drugs, supposedly produced at lower production cost? This justifies the apprehension about their quality in the minds of the clinicians and patients. There exists a widespread belief among people and dispensing chemists that a branded product is better in terms of quality and safety than the generic.[10,11]
Many reports comparing the effectiveness of branded generics and their branded counterparts are available. Many studies are conducted to test the therapeutic bio-equivalence of generic drugs even prior to marketing and there are number of published studies assuring the safety and efficacy of these generic drugs.[12-14] There are also many studies reporting that generic antibiotics behave differently from brand products against pathogenic microorganisms.[15,16] Doubts have also been raised about the efficacy of generic antibiotics, based on complaints from the medical community reported in the literature and at international meetings.[17] Farzana et al. have also reported comparable values of Minimum Inhibitory Concentrations for the local and multinational brands of 1st, 2nd and 3rd generation cephalosporins against clinical isolates of S. aureus.[18] A study carried out in Pakistan, showed no difference of in-vitro antibacterial activity of Ceftriaxone, even for the brand having the lowest MRP.[19] Another study by Bashir et al. reported that the multinational brands of Cephradine had better zones of inhibition than local Pharmaceutical companies. However, the difference was not statistically significant.[20]
Our study has shown that the branded and branded generic pairs of the same antibiotic had equal antimicrobial activity when tested in vitro. Comparable antimicrobial activity in vitro may however, get compromised by in adequate bioavailability of the formulation, resulting in inadequate antimicrobial activity in vivo. This aspect of comparison has not been addressed in our study. This limitation of our study needs to be taken care of by further studies. Such robust proof generated about equal effectiveness of these and other such pairs would make these products available at whatever cost benefit they offer to the patients. The other important point highlighted from our study and many others [18,19] is the tremendous difference in cost benefit to the retailers as compared to that for the patients. This demands that appropriate steps be taken to ensure that the cost benefit with the use of generics reaches the patients in appropriate amounts. Measures like defining the relative MRPs for branded and branded generic formulations of the same drug can be a step in this direction.
Conclusion
Our study revealed that the 3 branded and branded generic cephalosporins were equieffective in vitro. More elaborate studies would be needed to establish equal efficacy in vivo.
Measures are needed to ensure that the cost benefit does not remain restricted to the retailers and is also available to the patients in appropriate proportion.
References
- Singh P, Marwaha RK, Nanda A. A Comparative Evaluation of Branded and Generic Versions of Levocetrizine and Cephalexin. Asian Journal of Pharmaceutical Technology & Innovation 2014;02:09.
- Singal GL, Nanda A, Kotwani A. A comparative evaluation of price and quality of some branded versus branded-generic medicines of the same manufacturer in India. Indian J Pharmacology 2011;43:131-6.
- Johnson T.Available from: http://www.cfr.org/drugs/debate-over-generic-drug-trade/p18055(Updated: August 3/11).
- Biswas R, Chatterjee P, Mundle M. Prescribing habits of physicians in medical college, Calcutta. Indian J Community Med 2000;25:161-5.
- Manual on Antimicrobial Susceptibility Testing (Under the auspices of IndianAssociation of Medical Microbiologists) by Dr.MKLalitha, Vellore, Tamil Nadu(Last accessed on 21/1/16)
- Performance Standards for Antimicrobial Disk Susceptibility Tests, M100-S23, CLSI 33.
- EUCAST. Breakpoint tables for interpretation of MICs& zone diameters, version 4.0, valid from 2014.
- Pahwa N, Singal GL, NandaA. Quality of generics versus branded products for Cetrizine hcl tablets: A case study. The Indian Pharmacist 2010;16-22.
- Singal GL,Nanda A. A comparative study of branded versus branded generics in India. International Journal of Pharmacy & Technology 2010;2:960-8.
- Shafie AA, Hassali MA. Price comparison between innovator and generic medicines sold by community pharmacies in the state of Penang, Malaysia. J Gen Med 2008;6:35-42.
- Figueiras MJ, Marcelino D, Cortes MA. People′s views on the level of agreement of generic medicines for different illnesses. Pharm World Sci 2008;30:590-4.
- Vetchy D, Vetcha M, Rabiskova M, Gryczova E, Bartosikova L. Comparison in vitro felodipine release rate from the original versus generic product with controlled release of the drug. Medicina (Kaunas) 2007;43:326-31.
- Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Medication Adherence and the Use of Generic Drug Therapies. The American journal of managed care 2009;15:450.
- Reisman M. Generic Drugs: Caveat Emptor! (And Other Advice from Cyberspace). Pharmacy and Therapeutics 2010;35:215.
- Garyj M, Amya W, Helios S, Ronaldn J. Expanded studies of piperacillin/Tazobactam formulation: variation among branded product lots and assessment of 46 generic lots. Diagnostic Microbiology and Infectious Disease 2009;65:319-22.
- Zuluaga AF, Agudelo M, Rodriguez CA, Vesga O. Application of microbiological assay to determine pharmaceutical equivalence of generic intravenous antibiotics. BMC Clinical Pharmacology2009;9:1.
- Silva E, Diaz JA, Arias MJ, Hernandez AP, Torre A.Comparative in vitro study of the antimicrobial activities of different commercial antibiotic products for intravenous administration. BMC Clinical Pharmacology 2010;10:3.
- Farzana K, Noreen S, Nasir B,Azhar S, Mumtaz A. Comparative analysis of minimum inhibitory concentration of various brands of cephalosporin against clinical isolates of Staphylococcus aureus. Scientific Research and Essays 2011;6:6428-34.
- Masood H, Naqvi SB,Aslam N. Cost effective analysis of different brands of ceftriaxone available in karachi, pakistan Pakistan. Journal of Pharmacology 2008;25:13-9.
- Bashir S, Akhtar S, Hussain S, Malik F. Appraisal of multifarious brands of Cephradine for their in vitro antibacterial activity against varied microorganisms.Pak J Pharm Sci 2013;26:953-9.


