Assessment of HAART Clinical Outcomes and Associated Direct Costs in a Cohort of Patients Receiving Treatment at a Tertiary Healthcare Facility in North Central Nigeria
2 Federal Medical Centre, Keffi, Nassarawa State, Nigeria
Citation: Onah PO, Uthman UR. Assessment of HAART Clinical Outcomes and Associated Direct Costs in a Cohort of Patients Receiving Treatment at a Tertiary Healthcare Facility in North Central Nigeria.J B Clin Pharma 2017;8:216-220.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@jbclinpharm.org
Abstract
Background: The introduction of HAART has produced dramatic clinical improvements and quality of life in patients living with HIV/AIDS. Financial access to healthcare services is critical for survival. Currently little is known about direct costs to patients before and during regular access to care services. In the absence health insurance direct costs can be a significant barrier to financial access to care services. Objectives: To assess improvements in CD4 cell count and viral load and also determine direct costs associated with various HAART regimens as well as affordability of direct costs. Methods: This cross sectional study has both retrospective and prospective components. A total of 867 out of 5000 case notes that met inclusion criteria were selected by systematic random sampling. Direct costs were obtained from relevant departments and structured questionnaire. Data were entered into SPSS 20 and analyzed using one way anova with post hoc, student t test and Chi square as appropriate. P values ≤ 0.05 were considered significant. Results and Discussion: The most prescribed regimens were those containing Zidovudine+Lamivudine+Nevirapin e (Regimen I) and Tenofovir+Lamivudine/Emtricitabine+Efavirenz (Regimen IV) accounting for 38.4% and 49.1% respectively. Improvement in CD4 and viral load is significant across all regiments. The mean direct costs ranged between US$182.9-504.5 per encounter, which makes it highly unaffordable to majority of patients. Conclusion: Clinical improvement across all the HAART regimens is significant. Direct costs are highly unaffordable and this may impact negatively on access to care services.
Keywords
HIV/AIDS, direct cost, affordability, HAART
Introduction
Background
The introduction of highly active antiretroviral therapy (HAART) has dramatically improved favorable clinical outcomes, prognosis and quality of life of patients with HIV/AIDS. [1] Expansion of access to antiretroviral drugs has improved significantly over the last decade; patients even in rural communities are increasingly receiving free treatment. HIV/AIDS treatment has produced sustained viral suppression, reduction in incidence of opportunistic infections and significantly improved clinical outcomes. [2] Typically HAART regimen consist of three to four antiretroviral drugs and may include nucleoside reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), protease inhibitors (PI), Fusion inhibitors and integrase inhibitors. Despite the positive impact of HAART on clinical outcomes of treatment, patients on HAART have increased risk of costly adverse drug reactions which can potentially limit treatment options in the future. [3]
It is generally acknowledged that providing free antiretroviral drugs is an important step in HIV treatment. However other components of medical care costs can become important barrier to patients and their ability to afford costs of care. [4] While literatures abound with studies on cost effectiveness of antiretroviral regimens, little is known about direct costs to patients prior to accessing HIV/AIDS treatment services. These direct costs generally referred to as out of pocket expenditure is not covered by insurance. In many developing countries like Nigeria medical insurance is largely unavailable to the poor and the most vulnerable groups, consequently patients bear all costs incurred outside of antiretroviral drugs. Patients have been reported to spend twice the subsidized cost of antiretroviral drugs as out of pocket expenditure. [5]
In the early days after introduction of HAART cost of medical care began to decline primarily due to reductions in opportunistic infections and hospitalization, [6,7] however direct costs before patients can access care remained largely under studied. Improved access to HAART has dramatically improved in the last decade in Nigeria; poverty rate among the most affected population has worsened. Consequently the ability of patients to afford non HAART medications and services is an issue of concern to care givers.
Improvement in clinical outcomes of HAART is predicated on two surrogate markers namely CD4 cell count and viral load and some believe that there is a direct relationship between these markers and medical costs. Recent reports tend to suggest that HIV/AIDS care has moved from in-patient to out-patient care and patients are being placed on therapy early on in the disease,[8,9] so both direct and indirect costs are expected to rise over time. The rise in cost is particularly important for patients who often need to be switched from one regimen to another either due to resistance, adverse drug reactions, pregnancy or presence of co-morbidities all of which tend to increase direct costs of care. [10]
The incidence of adverse drug reactions is common in antiretroviral therapy and several reports have highlighted its association with increased morbidity and mortality. Adverse drug reactions are account for 5-10% of medical healthcare costs. [11] The medical costs associated with adverse drug reactions arise from hospitalization and long hospital stay[12] and reduction in the quality of life. While there is evidence as to the cost effectiveness of HAART,[13,14] the recent changes on guidelines that favor early initiation of treatment comes with increased cost burden on patients often from routine monitoring, hospital visits, non ARV medications etc. [15,16]
In a review[17] noted that direct costs of care are not widely reported particularly in poor resource settings. Direct costs can include but not limited to non ARV drugs, consultation, laboratory tests, hospital visits, transportation, special diet, co-morbidities and hospitalizations which are all added costs to patients. For instance non-infectious comorbidities like cardiovascular diseases, hypertension, renal failure and diabetes mellitus increases cost of care due to increased risk of hospitalization and mortality of patients on HAART. [18] These diseases often produce complex pathology requiring more care with its associated costs. [19]
In a developing country like Nigeria, direct and indirect costs can be a considerable burden to patients and care givers particularly in the face of extreme poverty. Besides, inability to pay for essential services as at when needed can hinder access and pose a huge challenge with adherence. Rural dwellers who generally live below the poverty line can hardly afford transportation costs, hospital services and non ARV drugs with far reaching implications for quality of care.
Objectives
1. To determine direct costs to patients on HAART regimens.
2. To assess improvement in CD4 cell count and viral load.
3. To identify any relationship between direct costs and antiretroviral regimen.
4. To determine affordability of patients out of pocket expenditures.
Methods
Setting;
The study was carried out in the outpatient department of the HIV/ AIDS clinic of federal medical centre Keffi, a referral hospital.
Study design
This is a cross sectional retrospective and prospective study design. Case notes of 5000 patients were selected by systematic random sampling for the study.
Sample size
This was determined by using Taro Yamanes’ formula, though higher sample size of 867 who met inclusion criteria was evaluated.
Data collection
A total of 867 case notes were selected using systematic random sampling using a sampling interval of 13. The selected medical records were reviewed and information on HAART regimen, hospitalization, adverse drug reactions, hospital visits, co-morbidities, opportunistic infections, viral load, CD4, non ARV drugs, duration on HAART regimens and other relevant information were extracted and entered into a specially designed data collection form. Cost of services, drugs, laboratory tests and hospitalization were obtained from the relevant departments of the hospital. Patient data were extracted and then twice at three month intervals making a total of one year.
A pretested questionnaire was self-administered to randomly selected patients during their routine hospital visits after informed consent was sought and obtained. This provided information on demographic data, distance to facility, transportation cost and other out of pocket expenditures.
Inclusion criteria
1. Patient must on the regimen for at least three months
2. Pregnant women and those below 18 years were excluded
3. There will no switch of regimen within the study period
4. Records of patients less than six months are not included
Data analysis
The data was collated and entered into SPSS 20 for descriptive and inferential statistics. Graphpad Instat 2.0 was also used inferential statistic where appropriate. Inferential statistics included one way anova with post hoc test, Student t test and Chi square as appropriate. P value ≤ 0.05 was considered statistically significant.
Ethical approval
This was sought and obtained from the health research ethics committee of the Federal medical centre, Keffi, Nassarawa State.
Results and Discussion
Highly active antiretroviral therapy has demonstrated good clinical outcomes for patients in not only producing significant improvements in CD4 counts and reduction in viral load, but also improvement in longevity and quality of life. As HAART becomes increasingly available to patients in extremely poor settings, direct costs to patients will become an important aspect of consideration for accessibility. The current guideline emphasized the need to place patients on HAART early in the course of the disease which is expected to increase out of pocket expenditure for patients. These cost borne by patients range from costs associated with laboratory tests, hospitalization, and treatment of adverse drug reactions, others include non ARV drugs, transportation and pharmacy services. Most patients in the study were mostly treated on outpatient basis, so while cost of hospitalization has significantly decreased over time, other direct costs have remained high and some cases increased. [20]
In Nigeria HAART that is provided in government healthcare facilities are free, so also are routine CD4 count and viral load tests. However all other costs including those for medications to treat opportunistic infections, laboratory services, transportation costs and treatment of drug reactions and co-morbidities are entirely borne by patients. This is where the problem lies for most patients particularly for the unemployed and those on the lowest rungs of socioeconomic ladder. Data from this study showed that about 65.9% of patients are above 36 years and females constitute the highest percentage with 58.2% [Table 1]. The most prescribed HAART regimens are I consisting of Zidovudine+Lamivudine+Nevirapne and regime IV consisting of Tenofovir+lamivudine/Emtricitabine+Efavirenz accounting for 34.8 and 49.9% of all prescriptions respectively [Table 2]. This is in contrast to other reports [21] in which Tenofovir+Emtricitabine+Efavirenz being the most prescribed regimen. The differences are likely due to the fact that selection of antiretroviral regimen is largely based on country specific guidelines and whether or not patients are being switched from one regimen to another. HAART regimen may be changed where there is failure of virologic response, adverse drug reactions and presence of co-morbidity. The change from regimen I to IV found in this study is largely due to poor virologic response and adverse reactions to Nevirapine based regimen. This is similar to other studies [22] which reported that Nevirapine based combination therapy caused adverse drug reactions in 36.7% as against 16.1% of Tenofovir based therapy. A number of other reports however noted that up to 30% of patients will require change in regimen within one year primarily due to toxicity, pregnancy, drug interactions, co-morbidities and viral drug resistance. [23] While it has been suggested that factors that generally influence choice of regimen are poorly understood, a retrospective cohort study indicated that both clinical and socioeconomic factors influence choice of HAART regimen and this is consistent with the results of this study. [24]
| Regimen I: Zidovudine+Lamivudine+Nevirapine Regimen II: Zidovudine+Lamivudine+Emtricitabine+Efavirenz Regimen III: Tenofovir+Lamivudine+Emtricitabine+Nevirapine Regimen IV: Tenofovir+Lamivudine/Emtricitabine+Efavirenz Regimen V: Azatanavir+Ritonavir+Zidovudine+Lamivudine+Emtricitabine Regimen VI: Lopinavir+Ritonovir+Zidovudine+Lamivudine+Emtricitabine |
Table 1: Available highly active antiretroviral regimens
| Variables | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Age (years) | n=301 | n=26 | n=17 | n=433 | n=23 | n=67 |
| Monthly income | 40.6 ± 10.6 | 31.5 ± 4.8 | 38.3 ± 4.7 | 37.1 ± 6.9 | 30.6 ± 7.7 | 39.5 ± 7.4 |
| (US$) | 76.8 ± 1.9 | 58.6 ± 1.1 | 98.9 ± 59.9 | 81.0 ± 63.2 | 57.9 ± 33.3 | 54.3 ± 35.9 |
| Gender | ||||||
| Male | 114 | 11 | 8 | 191 | 10 | 28 |
| Female | 187 | 15 | 9 | 242 | 13 | 39 |
| Occupation | ||||||
| Civil servant | 45 | 6 | 5 | 70 | 8 | 5 |
| Unemployed | 201 | 13 | 10 | 324 | 14 | 48 |
| Others | 55 | 7 | 1 | 185 | 1 | 14 |
| Marital status | ||||||
| Married | 217 | 14 | 12 | 386 | 15 | 52 |
| Single | 48 | 5 | 1 | 17 | 2 | 7 |
| Others | 36 | 7 | 4 | 30 | 6 | 8 |
Table 2: Demographic factors of patients
The results of this study indicate that there was general improvement in CD4 count across the entire six regimens, [Table 3] in particular patients on regimen I-IV had statistically significant rise in CD4 counts within the one year study period. This is consistent with other studies and once again demonstrates the appropriateness of these drug combinations in this setting. [25,26] Modest rise CD4 count found in patients on regimen V and VI is partly due to the fact that CD4 count has likely peaked before patients were switch to these second line regimens. A similar pattern was observed for viral load where most patients had almost undetectable viral load except for patient on regimen I [Table 4]. This observation is due to the fact that most new HAART patients were on regimen I, in spite of this observation the virologic response is quite high.
Direct cost on patients for all healthcare services offered to patients range between $182.9-504.4 per encounter [Table 5]. There are significant differences in direct costs between the regimens, for instance patients on regimen V spend more on hospitalization, bacterial opportunistic infections and adverse reactions [Table 6]. This is may be due to the fact that patients on this second line regimen are more likely to have co-morbidities and age related diseases. [27] In contrast patients on regimen I-IV spend less on hospitalization, adverse drug reactions and bacterial opportunistic infections. Majority of patients on first line HAART regimens have rapid improvement in clinical symptoms and this enables treatment on out-patient basis after short hospitalization period thus less direct cost. [1] The longer the patient is on HAART the more direct cost will rise accordingly. [28,29]
| Variable | I | II | III | IV | V | VI | P value |
|---|---|---|---|---|---|---|---|
| n=301 | n=26 | n=433 | n=17 | n=23 | n=67 | ||
| HAART(years) | 4.1 ± 1.9 | 2.8 ± 1.7 | 4.3 ± 1.7 | 4.3 ± 1.3 | 3.5 ± 2.1 | 5.7 ± 1.5 | <0.0001 |
| Co-morbidities (n) | 2.7 ± 1.2 | 3.3 ± 1.7 | 2.5 ± 1.1 | 4.1 ± 1.4 | 3.1 ± 1.1 | 1.2 ± 1.3 | <0.0001 |
| Hospital visits (n) | 4.7 ± 1. | 5.6 ± 1.2 | 4.7 ± 0.1 | 5.3 ± 1.1 | 4.7 ± 1.7 | 4.0 ± 1.6 | <0.0001 |
| Distance (Km) | 5.6 ± 4.5 | 4.5 ± 2.3 | 7.7 ± 4.4 | 7.3 ± 6.2 | 6.7 ± 4.2 | 6.1 ± 4.5 | 0.0006 |
| Drugs/prescription (n) | 4.3 ± 1.7 | 4.5 ± 2.3 | 4.6 ± 2.1 | 4.5 ± 1.7 | 4.9 ± 1.9 | 4.6 ± 1.8 | 0.4622 |
| Fungal infections (n) | 3.4 ± 1.9 | 3.8 ± 2.1 | 3.6 ± 1.9 | 8.7 ± 2.1 | 4.1 ± 2.2 | 3.8 ± 2.1 | <0.0001 |
| Bacterial infections (n) | 4.3 ± 2.3 | 3.8 ± 1.9 | 3.9 ± 1.9 | 4.5 ± 3.2 | 3.4 ± 1.9 | 3.7 ± 1.8 | 0.0961 |
| Viral infections (n) | 3.7 ± 1.9 | 3.4 ± 1.6 | 2.7 ± 1.9 | 3.5 ± 1.8 | 3.2 ± 2.1 | 4.1 ± 2.2 | 0.0533 |
| ADRs | 4.3 ± 1.7 | 4.5 ± 1.6 | 4.7 ± 2.1 | 4.5 ± 1.4 | 4.9 ± 1.9 | 4.6 ± 1.8 | |
| Average ADRs (n) | 1.8 | 1.4 | 1.8 | 2.0 | 2.1 | 2.2 | |
| Hospitalization (n) | 26 | 7 | 5 | 25 | 7 | 13 |
Table 3: Patient variables and HAART regimen
| Months | |||||||
|---|---|---|---|---|---|---|---|
| Regimen | N | 0 | 3 | 6 | 9 | 12 | P value |
| I | 301 | 492 ± 271.5 | 509.5 ± 259.8 | 558.6 ± 284.2 | 573.8 ± 253.4 | 615.1 ± 174 | <0.0001 |
| II | 26 | 578.2 ± 304.1 | 618.2 ± 278.6 | 702.7 ± 273.1 | 741.3 ± 229.2 | 790 ± 128.3 | 0.0166 |
| III | 17 | 572.9 ± 253.2 | 575 ± 150.6 | 643.7 ± 267.3 | 750 ± 125 | 774 ± 214.4 | 0.0112 |
| IV | 433 | 455.2 ± 274.8 | 587.2 ± 257.1 | 587.8 ± 226.5 | 626 ± 270 | 625.9 ± 240.3 | <0.0001 |
| V | 23 | 533.1 ± 239.4 | 564.2 ± 243.5 | 693.4 ± 288.2 | 671.9 ± 251.1 | 686 ± 234.3 | 0.0925 |
| VI | 67 | 532.1 ± 261 | 540.1 ± 231.8 | 593.7 ± 235.5 | 654.8 ± 200.4 | 701.6 ± 177.6 | 0.7112 |
Table 4: Mean increase in CD4 count
| Regimen | Months | ||
|---|---|---|---|
| 6 | 12 | % reduction | |
| I | 4,488 ± 344.9 (n=162) | 1048 ± 61.7 (n=18) | 88.9 |
| II | 1466 ± 187.4 (n = 14) | <50 | 100 |
| III | 2814.6 ± 201.8 (n=11) | <50 | 100 |
| IV | 2466.3 ± 187.4 (n=289) | 89.3 ± 27.6 (n=10) | 96.5 |
| V | 872.4 ± 103.5 (n=13) | <50 | 100 |
| VI | 649.3 ± 88.4 (n=28) | <50 | 100 |
Table 5: Percentage reduction in mean viral load
| Variable | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| n=301 | n=26 | n=17 | n=433 | n=23 | n=67 | |
| Transport | 16.2 ± 5.8 | 6.1 ± 3.5 | 7.7 ± 3.7 | 21.1 ± 17.3 | 21.4 ± 15.2 | 7.8 ± 3.7 |
| Hospital services | 7.2 ± 3.5 | 7.9 ± 4.3 | 8.2 ± 3.6 | 8.5 ± 4.9 | 9.3 ± 3.3 | 7.4 ± 3.1 |
| Adverse reactions | 47.3 ± 7.0 | 48.7 ± 14.7 | 61.1 ± 16.8 | 63.1 ± 3.9 | 60.2 ± 7.5 | 48.3 ± 5.9 |
| Hospitalization | 72.5 ± 9.2 | 92.4 ± 7.9 | 79.6 ± 7.5 | 89.6 ± 5.5 | 80.9 ± 4.9 | 64.9 ± 6.2 |
| Laboratory services | 34.2 ± 16.4 | 44.9 ± 26.7 | 44.6 ± 19.3 | 35.7 ± 21.3 | 36.9 ± 21.5 | 27.2 ± 16.7 |
| Non ARV drugs | 30.3 ± 16.5 | 50.4 ± 25.5 | 43.7 ± 17.1 | 41.4 ± 23.8 | 42.8 ± 21.5 | 36.9 ± 17.8 |
| Special diet | 5.4 ± 3.1 | 6.9 ± 4.3 | 5.9 ± 3.4 | 6.2 ± 3.6 | 6.3 ± 3.8 | 5.7 ± 3.1 |
| Bacterial infections | 28.2 ± 9.6 | 29.6 ± 11.9 | 25.4 ± 9.8 | 33.6 ± 10.0 | 31.9 ± 10.5 | 20.6 ± 8.4 |
| Fungal infections | 32.1 ± 12.2 | 38.9 ± 12.4 | 27.6 ± 10.2 | 32.7 ± 11.3 | 28.6 ± 12.5 | 23.8 ± 8.9 |
| Viral infections | 30.1 ± 9.9 | 35.7 ± 12.6 | 33.4 ± 10.4 | 36.1 ± 9.4 | 34.2 ± 30.7 | 25.6 ± 11.5 |
| Total | 303.5 ± 93.4 | 337.2 ±103.8 | 361.5 ± 123.6 | 368 ± 136.4 | 352.5 ± 133.3 | 268.2 ± 85.3 |
Table 6: HAART related out of pocket expenditure per hospital visit ($)
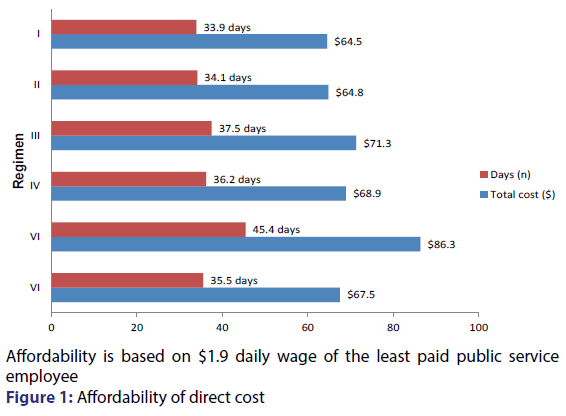
Most non ARV expenditure in Nigeria is paid as out of pocket expenditure by patients or relatives; this can present significant barrier to accessibility and adherence to recommended care services. The impact of direct cost of HIV/AIDS treatment often takes significant proportion of family income. [30,31] The results of this study indicate that most of non ARV direct costs are unaffordable by patients majority of who live below the official poverty line. The minimum wage for the least paid government worker in Nigeria is $57 per month using the official exchange rate of 315 naira to the dollar; it would take more than one month wage to cover direct cost of care per hospital encounter [Figure 1]. It was earlier reported [32] that HIV/AIDS patients are five times more likely to spend on healthcare services than HIV negative patients. Direct cost factors such as long distance to service delivery points, transportation cost, service provider fees etc. have first to be paid by patients before they access care. [33,34] This further complicates problems of affordability and those who cannot afford to pay may not likely receive the quality of care that is desired.
Conclusion
Direct cost to patients on HAART is a significant source of economic burden and is largely unaffordable by most patients. While there is significant improvement in HIV/AIDS surrogate markers across the regimens, direct cost associated with non ARV services are very high. Patients on second line regimen are more likely to spend more hospitalization due to adverse drug reactions and co-morbidities compared to those on first line regimens. Non ARV care services are highly unaffordable which raises concerns about financial access and eventually overall quality of care.
Disclosures
The study was funded from personal resources of the authors and there are no issues of conflict of interest.
Guarantor
I Dr. Paul Otor Onah responsibility for the integrity of this research from inception to publication.
REFERENCES
- Freedberg KA, Losina E, Weinsten MC. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 2001;344:824-31.
- Schackman BR, Gebo KA, Walensky RP. The lifetime cost of human immunodeficiency virus care in the United States. Med Care 2006;44:900-17.
- Poornima P, Rajesh R, Sudha V, Varma DM. Assessment of hematological ADRs to antiretroviral therapy in HIV positive patients at Kasturba Hospital, Manipal. BMC infect Dis 2012;12:55.
- Obinna O, Nkem D, Chinwe C, Uzochwube B. Examining catastrophic costs and benefits of incidence of subsidized antiretroviral treatment (ART) programme in south eastern Nigeria. Health policy 2008;90:223-9.
- Apanga S, Damien P, Adjei G. Estimating the cost to rural ambulatory HIV/AIDS patients on highly active antiretroviral therapy (HAART) in rural Ghana: a pilot study. Pan African Med J 2012;12:21.
- Gebo KA, Chaisson RE, Folkeme JG. Cost of medical care in an era of highly active antiretroviral therapy. AIDS 1999;13:963-9.
- Moulton Y, Alfendari S, Valette M. Impact of protease inhibitors on AIDS defining events and hospitalization; 10 French AIDS reference centers. AIDS 1997;10:101-5.
- Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet 2010;376:49-62.
- Thompson MA, Abong JA, Hoy JF. Antiretroviral treatment of adult HIV infection: 2012 recommendation of the international antiviral society-USA panel. JAMA 2012;308:387-402.
- Veronica JW, Steven F, Atanacio VM. Factors influencing global antiretroviral procurement prices. BMC public health 2009;9:6.
- Hartmut B, Krentz M, Christopher A, John G. The changing direct cost of medical care for patients with HIV/AIDS 1995-2001. Canadian medical association J 2003;169:106-10.
- Thiagu R, Surulivelrajana M, Vasudera G, Asha L, Rajesh V. Cost of adverse drug reactions in a south Indian tertiary care teaching hospital. J Clin pharmacol 2012;52:559-65.
- Stoll M, Claes C, Shultze E. Direct costs for the treatment of HIV infection in a German cohort after the introduction of HAART. Eur J Med Res 2002;7:463-71.
- Miners HH, Sabin CA, Truemen P. Assessing the cost effectiveness of HAART for adults with HIV in England. HIV Med 2001;2:52.
- Bozzette SA, Joyce G, McCaffrey DF. Expenditures for the care of HIV infected patients in the era of highly active antiretroviral therapy. N Engl J Med 2001;344:817-31.
- Walensky RP, Weinstein MC, Kimmel AD. Routine human immunodeficiency virus testing: an economic evaluation of current guidelines. Am J Med 2005;118:292-300.
- Levy AR, James D, Johnston KM, Hogg RS, Harrigan PR. The direct costs of HIV/AIDS care. Lancet Infect Dis 2006;6:171-7.
- Deeks SG, Philips AN. HIV infection, antiretroviral treatment, ageing and non AIDS related morbidity. BMJ 2009;338:288-92.
- Gebo KA, Flieshman JA, Conviser R. HIV research network; contemporary costs of HIV healthcare in the HAART era. AIDS 2010;24:2705-15.
- Yazdanpanah Y, Goldie SJ, Losina E. Lifetime cost of HIV care in France during the era of highly active antiretroviral therapy. Antiviral therapy 2002;7:257-66.
- Luigia E, Stefan E, Hansjakob F. Choice of initial combination antiretroviral therapy in individuals with HIV determinants and outcomes. Arch Intern Med 2012;172:1313-21.
- Radhakrshman R, Sudha V, Danturulu MV. Evaluation of direct cost of adverse drug reaction to highly active antiretroviral therapy in Indian human immunodeficiency virus positive patients. Clin Res HIV/AIDS and prevention. 2014;1:12-22.
- Elzi L, Marzolini C, Furrer H. Swiss HIV cohort study treatment modification in human immunodeficiency virus infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arh Intern Med 2010;170:57-65.
- Brouwer ESNS, Eron JJ. Influence of patient baseline clinical and demographic characteristics on choice of initial antiretroviral regimen; evidence of channeling drugs in HIV clinical care. Abstract TUPEB 2011;117.
- Hammer SM, Squires KE, Hughes MD. A controlled trial of two nucleoside analogues plus Indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic milliliter or less. N Engl J Med 1997;337:725-33.
- Smith C, Gerkus R, Walker S. Effective therapy has altered the spectrum of cause specific mortality following HIV seroconversion. AIDS 2006;20:741-9.
- Grabar S, Weiss I, Costagliola D. HIV infection in older patients in the HAART era. J Antimicrob Chemother 2006;57:4-7.
- Rizzadini G, Paolo B, Carenzi L. Cost effectiveness analysis of HIV treatment in the clinical practice of a public hospital in Northern Italy. Therap and Clin risk management 2012;8:377-81.
- Harling G, Wood R. The evolving cost of HIV in south Africa; changes in health care cost with duration of antiretroviral therapy for public sector patients. J acquir immune Defic syndr 2007;45:348-54.
- Ajay M, David C, Kunle O, Okonkwo P. Assessing the economic impact of HIV/AIDS on Nigerian households; a propensity score matching approach. AIDS 2008;22:95-101.
- Moon S, Van Leempt L, Durier N, Jambet E. Out of pocket costs of AIDS care in China; are free antiretrovirals enough? AIDS Care 2008;20:984-94.
- Jalal-Eddeen AS, Haruna IA. Barriers to HIV treatment in Nigeria. American J health Res 2015;3:305-9.
- Chindedza M, Mutseyekwa F, Chideme-Munodawafa A. Perceived barriers to accessing and achieving adherence in antiretroviral therapy among HIV patients at a rural mission hospital in Zimbabwe. Europ Sci J 2013;9:1857-81.
- Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E. A systematic review of individual and contextual factors affecting ART initiation, adherence and retention of HIV infected pregnant and post-partum women. Plos ONE 2014;9:E111421.