Antiviral activity of ancient system of ayurvedic medicinal plant Cissus quadrangularis L. (Vitaceae)
- *Corresponding Author:
- P. Balasubramanian
Department of Biotechnology, VELS University, Old Pallavaram, Chennai–600117, Tamil Nadu, India
E-mail: shaguftakhan17@rediffmail.com
Date of Received: 06-01-2010
Date of Modified: 15-01-2010
Date of Accepted: 26-01-2010
Available Online: 15-02-2010
Abstract
Abstract: Partially purified methanolic extract of Cissus quadrangularis (belonging to Vitaceae member, South Indian medicinal plant) have been explored for antiviral activity and their phytochemical characterisation. In vitro antiviral activity against HSV type1 and 2, and Vero cells at non-cytotoxic concentration were determined. HSV1 and HSV2 showed more sensitivity against the partially purified compound. Phytochemical analysis showed the presence of the Steroids and Terpenoids.
Keywords
Ayurvedic Cissus quadrangularis Antiviral Influenza virus HSV
Introduction
Cissus quadrangularis L is a medicinal plant belonging to family Vitaceae, the ancient system of medicine such as Ayurveda and used to treat various diseases and disorders. Within the past decade therapeutic options for viral infections have improved significantly, however, the emergence of resistant viruses as well. The further disposal of resistant strains is one reason for therapeutic failure [1–3]. Furthermore, many of the licensed drugs are toxic as well as being expensive [4], thus the search for potential sources for the development of new drugs is very important. Based on Ayurvedic and Siddha traditional herbal medicine, several antiviral studies were performed to detect active natural products in higher plants. In these studies different viruses were included, e.g. herpes simplex virus (HSV), feline imunodificidncy virus, coxsackie virus, influenza virus parainfluenza virus, respiratory syncytial virus, etc., [5–12]. Cissus used to treat various diseases and disorders, [13], antimicrobial, anti-osteoporotic, and antiviral activity [14–16]. Very meager research has been done in this plant against antimicrobial activity especially antiviral. Hence, the current study deals with the antiviral activity of partially purified compound of methanolic extract of Cissus quadrangularis has been observed in two in vitro virus HSV types 1 and 2 and also the phytochemical characterization was carried out.
Materials and Methods
Plant Material
Cissus quadrangularis stem were colleted from semi dry area where the plants were grown as a weed in various places in Tamil Nadu, India, which were pooled and processed for further study.
Extraction of plant material
The shadow air dried and powdered plant materials (20g) were extracted with methanol (MeOH) (400mL) in a Soxhlet extractor for 24 hrs filtered and evaporated to dryness under reduced pressure at 40°C. The residues obtained were re-dissolved in 100% DMSO and stored at–20°C until further use.
Purification of compound
The MeOH extracts separated using TLC with the solvent hexane and ethyl acetate, in the ratio of 1:9. The separated compounds were visualized. One of the compounds were scraped and removed for cytotoxicity and antiviral activity.
HPTLC / HPLC analysis HPTLC
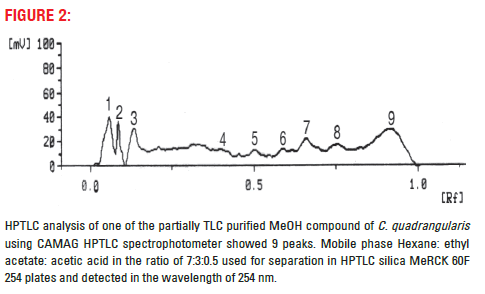
Sample was prepared by diluting 10 mg of partially purified fraction with 1 ml of the solvent, about 2 μl of the sample was loaded on a TLC plate using “CAMAG Linomat 4” applicator then the plate was kept in “CAMAG Twin Trough Chamber” of separation of compounds, with mobile phase Ethyl acetate, Hexane and Acetic acid in the ratio of 7:3:0.5. After the separation was over, the TLC plates were scanned using CAMAG TLC Scanner II at 254 nm wave length.
HPLC
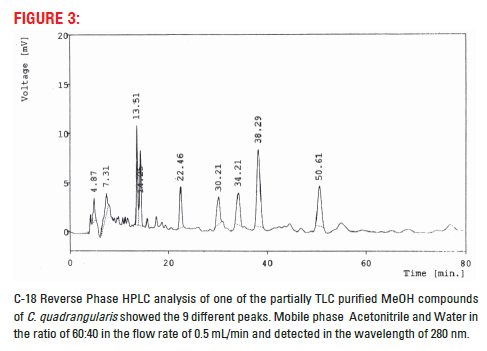
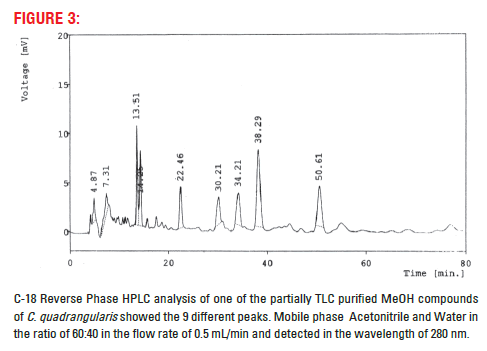
Partially purified compound was dissolved in acetonotrile. The sample was further separated through HPLC, C–18 (Phenomenox) analytical column using the mobile phase Acetonitrile:water in the ratio of 60:40 with the flow rate of 0.5 mL/min and detected in the UV detector at 280 nm.
Cells and Viruses
Vero cells (King Institute of Preventive Medicine, Tamil Nadu, India) were passaged in DMEM supplemented with 5% FBS. Herpes simplex virus type 1 and 2 were individually mixed with Vero cells and incubated at 36°C until the cytopathic effect develops. The cytopathic effected (virus in Vero cells) virus were estimated their TCID50 and stored at –70°C until use.
Cytotoxicity assay
The effect of the methanolic extract on the proliferation of Vero cells was determined in 96 well tissue culture plates and incubated at 37°C in humidified atmosphere supplied with 5% CO2. Confluent monolayers were incubated with two fold serial dilution of extracts (1:100, 200, 300, 400, 500 (100μg/mL)) in DMEM for 72 hrs. The 50% cell-inhibitory concentration (CC50) was determined by tryphan blue dye uptake assay using medium as a control.
Antiviral assay
Antiviral activity was determined using dye uptake assay [17]. HSV type 1 & 2 infected Vero cells were seeded in 96 well tissue culture plates at an initial cell concentration 60,000 cells/well and incubated in a humidified 5% CO2 atmosphere and preincubated with 100μL of serial dilution of partially purified compound of MeOH extract in the medium (1:100, 200, 300, 400 (100μg/mL)) in triplicate and control virus was kept for 15 min at same condition, and incubated at 36°C for 7 days.
Phytochemical Screening
The screening of the chemical constitutents was carried out in MeOH extracts using chemical methods and TLC. Lieberman Buchard Test for Steroids, Dragendroff’s test for Alkaloids, Feric solution test for Tannins, Fehling’s test for Reducing sugars, Ninhydrin test for Amino acid and proteins and for Terpenoids, Ammonium heptamolybdate and cericsulphate in sulphuric acid along with sample heated at 120°C and the appearance of blue indicate the presence of terpenoids were analyzed.
Statistical Analysis
The statistical significance P value of dilution of MeOH extracts C. quadrangularis activity against HSV 1 and 2 were calculated using statistical software SPSS for Windows 10.1. P value less than 0.05 was considered to be statistically significant
Results and Discussion
Partially purified compound of MeOH extracts of C. quadrangularis was screened for antiviral activity against HSV type 1 and 2 viruses (Fig. 1a&b). The antiviral activity was evaluated at concentrations that were non-toxic for the cell system. Cytotoxicity assay of TLC purified compound of MeOH extracts showed the cytopathetic effect in 1: 200 (Table 1) dilution. In the antiviral activity of partially purified compound of MeOHextracts of C. quadrangularis against HSV 1 and 2 showed were inhibited statistically significant (p<0.01) level at 1: 400 dilution (Table 2).
| Extract | CC | Wells 1:100 | 1:200 | 1:300 | 1:400 | 1:500 | |
|---|---|---|---|---|---|---|---|
| Methanol | 0 | 2 | 0 | 0 | 0 | 0 | |
CC – Cell Control; 0 – No effect; 2 – 2+ cytopathic effect
Table 1: Cytotoxicity assay of partially purified compound of methanol extracts of C. quadrangularis in Vero cells.
| Extract | CC | 1:100 | 1:200 | 1:300 | 1:400 | Virus |
|---|---|---|---|---|---|---|
| Methanol | 0 | 0 | 0 | 0 | 4** | HSV 1 |
| 0 | 0 | 0 | 0 | 4** | HSV 2 |
CC – Cell Control; 0 – No effect; 4 – 4+ cytopathic effect;
** Statistical significance p<0.01
Table 2: Antiviral activity of partially purified compound of methanol extracts of C. quadrangularis in HSV 1 and HSV 2 infected Vero cells.
Aqueous and MeOH extracts of its partner species of Cissus subaphylla and C. hamaderohensis and Undaria pinnatifida showed its activity against HSV [15,16,18] which confirm our experiments using partially TLC purified compound of methanol extracts of C. quadrangularis. This partially purified compound further studied for their purity and multiple compounds in its, 9 compounds were separated and detected in HPTLC. However, 1, 2, 3 and 9 compounds were separated distinctly where, 4, 5, 6, 7 and 8 compounds were not separated distinctly, these compounds may closely associate together (Fig. 2). In HPLC also showed 9 matching different compounds were separated. The result reveals that the partially TLC purified compound contain multiple compounds and associated together compounds (Fig. 3). Further study need to identify the single active compound against antiviral activity.
Figure 2: HPTLC analysis of one of the partially TLC purified MeOH compound of C. quadrangularis using CAMAG HPTLC spectrophotometer showed 9 peaks. Mobile phase Hexane: ethyl acetate: acetic acid in the ratio of 7:3:0.5 used for separation in HPTL C silica MeRCK 60F 254 plates and detected in the wavelength of 254 nm.
In phytochemical screening (Table 3) revealed the presence of general steroids and Terpinoids. Previously work carried out by number of investigators with these compounds posses anti-viral activity [16,19–21]. Melchart, et al. (1994) [22] proved that Echinacea species extracts through oral treatment protects experimentally infected the Influenza–A virus in mice. The immunomodulating action may triggered by antiviral activity compounds [23].
| Compound | Partially purified ethanol compound |
|---|---|
| Steroids | + |
| Terpenoids | + |
| Alkaloids | – |
| Flavonoids | – |
| Tannin | – |
| Amino acid/Protein | – |
| Sugar | – |
+ Present; – Absent
Table 3: Phytochemical analysis of partially purified compound of methanol extract of C. quadrangularis.
In conclusion, the present investigation showed that the partially purified compound confirms high antiviral activity. Further study has been continued of this partially purified compound, furthermore purified and to identify the active compound possessing antiviral activity in MeOH extracts and to formulate the drug against viral infection.
References
- Gadreau A, Hill E, Balfour Jr., HH, et al. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex virus from immunocompromised patients. Journal of Infectious Disease. 1998; 178: 29 – 303.
- Emery VC. Cytomegalovirus drug resistance. Antiviral Therapy. 1998; 4: 239 – 242.
- Safrin S, Cherrington J, Jaffe HS. Cidofovir: review of current and potential clinical uses. Advance Experimental Medical Biology. 1999; 458: 111–120.
- Pillay D, Emery VC, Mutimer D, et al. Guidelines for laboratory monitoring of treatment of persistent virus infections. Journal of Clinical Virology. 2000: 25: 73 – 92.
- Abad MJ, Guerra JA, Bermejo P, et al. Search for antiviral activity in higher plant extracts. Phytotherapy Research. 2000; 14: 604–607.
- Rajbhandari M, Wegner U, Jülich M, et al. Screening of Nepalese medicinal plants for antiviral activity. Journal of Ethnopharmacology. 2001; 74: 251–255.
- Schmitt AC, Ravazzolo AP, von Poser GL. Investigation of some Hypericum species native of Southern of Brazil for antiviral activity. Journal of Ethnopharmacology. 2001; 77: 239–245.
- Cos P, Hermans N, De Bruyne T, et al. Further evaluation of Rwandan medicinal plant extracts for their antimicrobial and antiviral activities. Journal of Ethnopharmacology. 2002; 79: 155–163.
- Li YL, Ma SC, Yang YT, et al. Antiviral activities of flavonoids and organic acids from Trollicus chinensis Bunge, Journal of Ethnopharmacology. 2002; 79: 365 – 368.
- Ooi LS, Wang H, Luk CW, et al. Anticancer and antiviral activities of Youngia japonica (L.) DC (Asteraceae, Compositae). Journal of Ethnopharmacology. 2004; 94: 117–122.
- Li Y, Ooi LSM, Wang H, et al. Antiviral activities of medicinal herbs traditionally used in Southern mainland China. Phytotherapy Research. 2004; 18: 718 –722.
- Vijayan P, Raghu C, Ashok G, et al. Antiviral activity of medicinal plants of Nilgiris. Indian Journal of Medical Research. 2004; 120: 24 – 29.
- Geissler PW, Harris SA, Prince RJ, et al. Medicinal plant used by Luo mothers and children in Bondo District, Kenya. Journal of Ethanopharmacol. 2002; 83: 39 – 54.
- Chidambara Murhy KN, Vanitha A, Mahadeva Swamy M, et al. An assenssment of the phytochemical and nutrient composition of the pulverized root of Cissus quadrangularis. Bioresearch. 2003; 1: 63 – 68.
- Mothana RAA, Lindequist U. Antimicrobial activity of some medicinal plants of the island Soqotra. Journal of Ethnopharmacology. 2005; 96: 177–181.
- Mothana RAA, Mentel R, Reiss C, et al. Phytochemical screening and antiviral activity of some medicineal plants from the island Soqotra. Phytotherapy Research. 2006; 20: 298 – 302.
- Nowotny A, Mentel R, Wegner U, et al. Antiviral activity of an aqueous extract of the cyanobacterium Microcystis aeruginosa. Phytotherapy Research. 1997; 11: 93 – 96.
- Cheng HY, Lin CC, Lin TC. Antiviral properties of prodelphinidin B–2 3’-o-gallate from tea leaf. Antiviral Chemistry and Chemotherapy. 2002; 13: 223 – 229.
- Du J, He ZD, Jiang RW, et al. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry. 2003; 62: 1235 – 1238.
- Wei F, Ma SC, Ma LY, et al. Antiviral flavonoids from the seeds of Aesculus chinensis. Journal of Natural Products. 2004; 67: 650 – 653.
- Bodinet C, Mentel R, Wegner U, et al. Effect of oral application of an immunomodulating plant extract on influenza virus type A infection in mice. Planta Medica. 2002; 68: 896 – 900.
- Melchart D, Linde K, Worku F, et al. Immunomodulation with Echinacea – a systematic review of controlled clinical trials. Phytomedicine. 1994; 1: 245 –254.
- Bodinet C, Freudenstein J. Effects of an orally applied aqueous-ethanolic extract of a mixture of Thujae occidentalis herba, Baptisiae tinctoriae radix, Echinaceae purpureae radix and Echinaceae pallidae radix on antibody response against sheep red blood cells in mice. Planta Medica. 1999; 65: 695 – 699.