Antiurolithiatic activity of Abelmoschus moschatus seed extracts against zinc disc implantation-induced urolithiasis in rats
- *Corresponding Author:
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: The commonly used techniques for removing renal calculi are associated with the risk of acute renal injury and increase in stone recurrence which indicates an urgent need for alternate therapy. Objectives: The aim was to evaluate the antiurolithiatic activity of Abelmoschus moschatus seed extracts in rats. Materials and Methods: Urolithiasis was induced by surgical implantations of zinc disc in the urinary bladders of rats. Upon postsurgical recovery, different doses of chloroform (CAM) and methanolic (MAM) extracts of A. moschatus seeds (viz., 100, 200 and 400 mg/kg body weight) were administered to disc implanted rats for the period of 7 days by the oral route. Antiurolithiatic activity was evaluated by measuring various dimensions of stones and estimating levels of various biomarkers in serum and urine samples. Results: A significant decrease in urinary output was observed in disc implanted animals, which was prevented by the treatment with extracts. Supplementation with extracts caused significant improvement in glomerular filtration rate and urinary total protein excretion. The elevated levels of serum creatinine, uric acid, and blood urea nitrogen were also prevented by the extracts. The extracts significantly reduced deposition of calculi deposition around the implanted disc. This antiurolithiatic potential is observed at all doses (100, 200, and 400 mg/kg) of MAM, whereas only higher dose (400 mg/kg) of CAM showed significant antiurolithiatic potential. Conclusion: The extracts of A. moschatus seeds possessed significant antiurolithiatic activity. The possible mechanism underlying this effect is mediated collectively through diuretic, antioxidant, and free‑radical scavenging effects of the plant.
Keywords
Abelmoschus moschatus, urolithiasis, zinc disc implantation
Introduction
Abelmoschus moschatus (family: Malvaceae) is cultivated in the tropical regions of Asia, Africa, and South America for its seeds which are used mostly for the isolation of fragrance components. [1] In India, it is found wild all over the hilly regions of Deccan and Karnataka and also at the foothills of the Himalayas. [2] A. moschatus has been extensively studied by various researchers for its biological activities and therapeutic potentials such as diuretic, antioxidant activity and free-radical scavenging, antiproliferative, antimicrobial, antilithiatic, hepatoprotective, memory strengthening, antidiabetic, hemagglutinating, antiageing, antidepressant, anxiolytic, anticonvulsant, hypnotic, and muscle relaxant activity. [3-14] In Indian indigenous system of medicine, seeds of A. moschatus are claimed to be useful for the renal calculi. Therefore, this study was undertaken to evaluate the antiurolithiatic activity of A. moschatus seed extracts against zinc disc implantation-induced urolithiasis in rats to support its folkloric claim.
Materials and Methods
Animals
Male Wistar albino rats weighing between 150 and 250 g were used in this study. They were procured from the National Institute of Biosciences, Pune, India. The animals were allowed to acclimatize for 10 days under standard conditions in an animal house approved by Committee for the Purpose of Control and Supervision of Experiments on Animals, India. The animals were given standard diet supplied by Nutrivet Life Sciences, Pune, India. The study protocol was approved by the Institutional Animal Ethics Committee (Ref. No.: MIP/IAEC/2012-13/M1/Appr/002) of the institute.
Chemicals and apparatus
Ketamine (Injection Aneket, Neon Laboratories Ltd., India), diazepam (Injection Calmpose, Ranbaxy Laboratories Ltd., India), ibuprofen (Tablet Brufen, Abbott India Ltd., India), and cystone (Tablet Cystone, Himalaya Drug Company, India) were used for this study. Apparatus such as the metabolic cages (New Neeta Chemicals, India), cold centrifuge (BioEra, India), and ultraviolet spectrophotometer (LabIndia, India) were used in the study.
Plant material and preparation of extract
The capsules of A. moschatus were collected in the month of November from the local region of Thane, Maharashtra, India. They were authenticated by Dr. A. S. Upadhye, Agharkar Research Institute, Pune, India. The capsules were dried in shade followed by drying of separated seeds in shade for 10–12 days. The dried seeds of A. moschatus were coarsely powdered, packed into soxhlet column, and extracted separately with chloroform and 70% v/v methanol solution for 22 h. The obtained chloroform (CAM) and methanolic (MAM) extracts of A. moschatus were evaporated at 45°C and then dried in an oven. The dried extracts were stored in airtight container.
Preliminary phytochemical analysis
Phytochemical screening of the CAM and MAM was performed to detect the presence of different classes of phytoconstituents by standard methods. [15,16] The qualitative results are expressed as (+) for the presence and (−) for the absence of phytoconstituents.
Test for carbohydrates (Molisch’s test)
Two drops of Molisch’s reagent (α-napthol in 95% ethanol) was added to 2 ml of the extract solution. The resultant solution is mixed and few drops of concentrated H2SO4 were added from the sides of the test tube. Appear of the violet ring at the junction was taken as a positive result for carbohydrates.
Test for protein with biuret reagent
2 ml of 4% NaOH solution and 4–5 drops of 1% CuSO4 solution was added to 3 ml of the extract solution. The presence of violet or pink color was indicative of the proteins.
Test for amino acids with ninhydrin reagent
A volume of 3 ml of extract solution and 3 drops of ninhydrin solution were mixed and heated in boiling water bath for 10 min. The appearance of purple or bluish color was taken as a positive result for the presence of amino acids.
Test for fats and oils (Spot test)
The dry powdered extract was pressed with filter paper. The formation of permanent stain on filter paper was considered as preliminary evidence of the presence of the fats and oils.
Test for steroids (Salkowski test)
About 0.5 g of the extract was dissolved in 3 ml of chloroform and filtered. 2 ml of concentrated H2SO4 was carefully added to the filtrate to form lower layer. A turbid reddish brown color at the interface was taken as a positive result for the presence of steroid glycosides.
Test for volatile oils (Stain test)
The dry powdered extract was pressed with filter paper. The formation of temporary stain on filter paper was considered as preliminary evidence of the presence of the volatile oils.
Test for cardiac glycosides (Killer–Killiani test)
About 5 mg of extract was treated with 1 ml of glacial acetic acid and 2–3 drops of 5% FeCl3 solution were added into the test tube. To this mixture 2 ml of conc. H2SO44 was added carefully along the sides of the test tubes. The formation of a reddish brown color at the junction of two layers and bluish green color to upper layer were considered as an indication of the presence of the cardiac glycosides.
Test for anthraquinone glycosides (Modified Borntrager’s test)
A volume of 2 ml of extract was mixed with 2 ml of 5% FeCl3 solution and 2 ml of 10% HCI solution. The resultant mixture was boiled in water bath for 5 min. Extract filtered hot, cooled, and extracted with 5 ml of chloroform. The chloroform layer was treated with equal volume of dilute NH3 solution. The formation of pinkish red color to ammoniacal layer was taken as a positive result for the presence of anthraquinone glycosides.
Test for cyanogenetic glycosides (Sodium picrate test)
Filter paper strip was soaked first in 10% picric acid and then in 10% sodium carbonate. The soaked filter paper was dried at 40°C in an oven. In a conical flask, moistened powdered extract was placed, and the conical flask was corked after placing the above filter paper strip in the slit in cork. The formation of brick red color to filter paper was considered as an indication of the presence of the cyanogenetic glycosides.
Test for coumarin glycosides (Fluorescence test)
About 5 mg of extract was dissolved in 5 ml of absolute ethanol and filtered. 5 ml of dilute NaOH was carefully added to the filtrate. The formation of blue or green fluorescence was taken as preliminary evidence for the presence of coumarin glycosides.
Test for saponins (Foam test)
A volume of 1 ml of the extract was diluted with distilled water to 20 ml and shaken in a graduated cylinder for 15 min. 1 cm layer of foam was considered as an indication of the presence of saponins.
Test for flavonoids (Shinoda test)
About 0.2 g of the extract was dissolved in 5 ml of 95% ethanol and heated. A chip (0.5 g) of magnesium metal was added to the mixture followed by the addition of 4–5 drops of concentrated hydrochloric acid. The occurrence of a red or orange coloration was taken as preliminary evidence for the presence of flavonoids.
Test for alkaloids with Mayer reagent
To 2 ml of extract solution, 4–5 drops of Mayer’s reagent (Potassium mercuric iodide solution) was added. The formation of a cream colored precipitate was considered as an indication for the presences of alkaloids.
Test for tannins and phenolic compounds with ferric chloride solution
About 0.5 g of extract was stirred with 10 ml of distilled water and then filtered. To 2 ml filtrate, 2 ml 5% FeCl3 solution was added. The formation of black or blue-green coloration or precipitate was taken as a positive result for the presence of tannins.
Antiurolithiatic activity of CAM and MAM in rats
The three dose levels (100, 200, and 400 mg/kg) of CAM and MAM were used for the evaluation of their antiurolithiatic effect in the study. [17]
Surgical implantation of zinc disc in bladders of rats
The implantations of zinc disc in the urinary bladders of rats were carried out by earlier reported method. [18] Rats were fasted for 8–10 h before the surgical procedure. However, they were orally administered with 4 ml of water (15–20 min prior to anesthesia) in order to dilate their urinary bladder. The rats were operated in sterile conditions under ketamine (80 mg/kg, i.p.) and diazepam (4 mg/kg, i.m.) anesthesia. The urinary bladders were exposed through a suprapubic incision, and a small cut was taken to open the lumen of the bladder. The zinc disc of average weight 20 ± 2 mg was inserted into the bladder, and the incision was closed by 1 or 2 stitches of absorbable sterile surgical sutures. The urinary bladder was pushed back into its earlier position. The muscular layer of the abdomen was separately sutured using sterile absorbable sutures. The skin incision was then stitched with sterile silk sutures. All operated rats were administered with ibuprofen (30 mg/kg, p.o.) on the day of surgery immediately after recovery from anesthesia and this dose was repeated after 24 h. The rats were treated topically with antibiotic dusting powder and allowed to recover for 3 days.
Experimental design
The completely recovered rats were randomly divided into nine groups containing six animals in each. Group I served as a sham-operated group (underwent same surgical procedure but without implantation of zinc disc) and received 5% w/v gum acacia solution (5 ml/kg, p.o.). Group II was zinc disc-implanted group and received 5% w/v gum acacia solution (5 ml/kg, p.o.). Group III was zinc disc-implanted and standard drug treated group, received cystone (500 mg/kg) for next 7 days. Group IV, V, and VI were zinc disc-implanted and CAM-treated groups, received CAM at doses of 100, 200, and 400 mg/kg, respectively, for next 7 days. Group VII, VIII, and IX were zinc disc-implanted and MAM-treated groups, received MAM at doses of 100, 200, and 400 mg/kg, respectively, for 7 days. The cystone, CAM, and MAM were suspended in distilled water using 5% w/v gum acacia and given after recovery once daily by oral route (5 ml/kg body weight) for 7 days.
Serum analysis
One hour after the last dose of treatment, blood was collected from retro-orbital sinus under ether anesthesia. Serum was separated by centrifugation at 10,000 ×g for 10 min and analyzed for creatinine, uric acid, and blood urea nitrogen (BUN) spectrophotometrically by using diagnostic kits (Beacon Diagnostic Pvt. Ltd., India).
Urine analysis
After blood collection, all animals were kept in individual metabolic cages for the collection of 5 h urine samples. The collected urine samples were measured for volume, total protein, and creatinine levels. The total protein and creatinine levels were estimated using diagnostic kits (Beacon Diagnostic Pvt. Ltd., India).
Stone estimations
After urine collection period, all animals were sacrificed by cervical dislocation. The urinary bladders were exposed, zinc disc along with adhered crystals was removed, and the weight of calculi was noted. The dimensions of zinc disc with adhered crystals were also determined by using Vernier caliper.
Statistical analysis
All the results were expressed as mean ± standard error of mean (SEM). The statistical significance was calculated using one-way analysis of variance followed by Dunnett’s comparison test and P < 0.05 was considered significant. [19]
Results
Extraction of plant material and preliminary phytochemical analysis
The yields of CAM and MAM were found to be 18.45 and 16.67% w/w, respectively. Phytochemical analysis revealed the presence of carbohydrates, fixed oil and fats, flavonoids, alkaloids, tannins, and phenolic compounds in the CAM, whereas MAM was found to contain carbohydrates, proteins and amino acids, flavonoids, saponins, tannins, and phenolic compounds [Table 1].
| Phytochemicals | Test | CAM | MAM |
|---|---|---|---|
| Carbohydrates | Molish’s test | + | + |
| Protein | Biuret test | − | + |
| Amino acids | Ninhydrin reagent | − | + |
| Fats and oils | Spot test | + | − |
| Steroids | Salkowski reaction | − | − |
| Volatile oils | Stain test | − | − |
| Cardiac glycosides | Killer–Killiani test | − | − |
| Anthraquinone glycosides | Modified Borntrager’s reagent | − | − |
| Cyanogenetic glycosides | Sodium picrate test | − | − |
| Coumarin glycosides | Fluorescence test | − | − |
| Saponins | Foam test | − | + |
| Flavonoids | Shinoda test | + | + |
| Alkaloids | Mayer’s test | + | − |
| Tannins and phenolic | Test with ferric chloride | + | + |
| compounds | solution |
+: Present, −: Absent, CAM: Chloroform extract of Abelmoschus moschatus, MAM: Methanolic extract of Abelmoschus moschatus
Table 1: Phytochemical screening of Abelmoschus moschatus extracts
Effect on urine volume
The mean urine volume of sham-operated animals was 2.48 ± 0.17 ml/5 h, which was significantly (F [8,45] =8.81, P < 0.001) decreased to 1.27 ± 0.11 ml/5 h in zinc disc implanted animals [Table 2]. Treatment of animals with cystone (500 mg/kg), higher dose of CAM (400 mg/kg), intermediate, and higher doses of MAM (200 and 400 mg/kg) caused a significant increase in the urine volume as compared to zinc disc implanted group [Tables 3 and 4].
| Parameters | Mean±SEM | P | |
|---|---|---|---|
| Sham-operated | Zinc disc-implanted | ||
| Urine | |||
| Volume (ml/5 h) | 2.48±0.17 | 1.27±0.11 | <0.001 |
| Creatinine (mg/dl) | 1.78±0.08 | 2.41±0.13 | <0.001 |
| Total protein (mg/5 h) | 1.07±0.11 | 3.10±0.21 | <0.001 |
| Serum | |||
| Creatinine (mg/dl) | 0.71±0.02 | 1.88±0.06 | <0.001 |
| Uric acid (mg/dl) | 1.24±0.04 | 3.29±0.10 | <0.001 |
| BUN (mg/dl) | 37.95±0.74 | 51.13±1.36 | <0.001 |
| Calculi | |||
| Weight (mg) | 00.00±0.00 | 78.32±5.65 | <0.001 |
| Diameter (mm) | 0.00±0.00 | 4.92±0.35 | <0.001 |
| Thickness (mm) | 0.00±0.00 | 5.85±0.23 | <0.001 |
Number of animals n=6. P<0.05: Significant. Values of zinc disc-implanted group were compared with the Sham-operated group. BUN: Blood urea nitrogen, SEM: Standard error of mean
Table 2: Effect of zinc disc implantation on urine, serum and dimensions of calculi in rats
| Parameters | Mean±SEM | P | CAM-100 mg/kg (mean±SEM) | P | CAM-200 mg/kg (mean±SEM) | P | CAM-400 mg/kg (mean±SEM) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Zinc disc-implanted | Cystone-500 mg/kg | ||||||||
| Urine | |||||||||
| Volume (ml/5 h) | 1.27±0.11 | 2.28±0.16 | <0.001 | 1.37±0.12 | >0.05 | 1.65±0.13 | >0.05 | 1.93±0.14 | <0.05 |
| Creatinine (mg/dl) | 2.41±0.13 | 1.80±0.10 | <0.001 | 2.17±0.12 | >0.05 | 2.06±0.11 | >0.05 | 1.97±0.09 | <0.05 |
| Total protein (mg/5 h) | 3.10±0.21 | 1.25±0.13 | <0.001 | 2.91±0.19 | >0.05 | 2.72±0.16 | >0.05 | 2.41±0.14 | <0.05 |
| Serum | |||||||||
| Creatinine (mg/dl) | 1.88±0.06 | 0.83±0.02 | <0.001 | 1.84±0.05 | >0.05 | 1.76±0.06 | >0.05 | 1.70±0.04 | <0.05 |
| Uric acid (mg/dl) | 3.29±0.10 | 1.34±0.04 | <0.001 | 3.17±0.09 | >0.05 | 3.05±0.09 | >0.05 | 2.95±0.09 | <0.05 |
| BUN (mg/dl) | 51.13±1.36 | 39.12±0.81 | <0.001 | 50.17±1.28 | >0.05 | 48.55±1.16 | >0.05 | 46.44±0.89 | <0.05 |
| Calculi | |||||||||
| Weight (mg) | 78.32±5.65 | 15.66±1.20 | <0.001 | 70.17±3.45 | >0.05 | 66.00±3.14 | >0.05 | 62.29±3.88 | <0.01 |
| Diameter (mm) | 4.92±0.35 | 1.70±0.30 | <0.001 | 4.40±0.35 | >0.05 | 3.97±0.31 | >0.05 | 3.88±0.33 | >0.05 |
| Thickness (mm) | 5.85±0.23 | 1.97±0.14 | <0.001 | 5.42±0.21 | >0.05 | 5.20±0.20 | >0.05 | 4.84±0.20 | <0.01 |
Number of animals n=6. P<0.05: Significant. Values of cystone and CAM-treated groups were compared with zinc disc-implanted group. BUN: Blood urea nitrogen, SEM: Standard error of mean, CAM: Chloroform extract of Abelmoschus moschatus
Table 3: Effect of chloroform extract of Abelmoschus moschatus on urine, serum and dimensions of calculi in urolithiasis-induced rats
| Parameters | Mean±SEM | P | MAM-100 mg/kg (mean±SEM) | P | MAM-200 mg/kg (mean±SEM) | P | MAM-400 mg/kg (mean±SEM) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Zinc disc-implanted | Cystone-500 mg/kg | ||||||||
| Urine | |||||||||
| Volume (ml/5 h) | 1.27±0.11 | 2.28±0.16 | <0.001 | 1.47±0.13 | >0.05 | 1.90±0.14 | <0.05 | 2.08±0.16 | <0.01 |
| Creatinine (mg/dl) | 2.41±0.13 | 1.80±0.10 | <0.001 | 2.00±0.10 | <0.05 | 1.93±0.10 | <0.05 | 1.88±0.09 | <0.01 |
| Total protein (mg/5 h) | 3.10±0.21 | 1.25±0.13 | <0.001 | 2.05±0.11 | <0.001 | 1.89±0.15 | <0.001 | 1.53±0.10 | <0.001 |
| Serum | |||||||||
| Creatinine (mg/dl) | 1.88±0.06 | 0.83±0.02 | <0.001 | 1.68±0.05 | <0.05 | 1.20±0.03 | <0.001 | 0.94±0.03 | <0.001 |
| Uric acid (mg/dl) | 3.29±0.10 | 1.34±0.04 | <0.001 | 2.96±0.09 | <0.05 | 2.55±0.07 | <0.001 | 1.62±0.06 | <0.001 |
| BUN (mg/dl) | 51.13±1.36 | 39.12±0.81 | <0.001 | 46.81±1.02 | <0.05 | 45.64±1.11 | <0.01 | 44.60±1.06 | <0.001 |
| Calculi | |||||||||
| Weight (mg) | 78.32±5.65 | 15.66±1.20 | <0.001 | 64.06±3.75 | <0.05 | 59.59±3.01 | <0.01 | 36.80±2.32 | <0.001 |
| Diameter (mm) | 4.92±0.35 | 1.70±0.30 | <0.001 | 3.78±0.37 | >0.05 | 3.37±0.27 | <0.01 | 2.17±0.27 | <0.001 |
| Thickness (mm) | 5.85±0.23 | 1.97±0.14 | <0.001 | 5.13±0.21 | <0.05 | 4.90±0.20 | <0.01 | 3.65±0.17 | <0.001 |
Number of animals n=6. P<0.05: Significant. Values of cystone and MAM-treated groups were compared with zinc disc-implanted group. BUN: Blood urea nitrogen, SEM: Standard error of mean, MAM: Methanolic extract of Abelmoschus moschatus
Table 4: Effect of methanolic extract of Abelmoschus moschatus on urine, serum and dimensions of calculi in urolithiasis-induced rats
Effect on urinary creatinine and total protein levels
Zinc disc implantation caused significant (F[8,45] =3.63, P < 0.001) increase in the urinary creatinine level from 1.78 ± 0.08 mg/dl to 2.41 ± 0.13 mg/dl [Table 2]. This increased urinary creatinine level was significantly reduced by the treatment with cystone (500 mg/kg), higher dose of CAM (400 mg/kg), and all three doses of MAM (100, 200, and 400 mg/kg) in a dose-dependent manner [Tables 3 and 4].
The mean urinary total protein excretion of sham-operated animals was 1.07 ± 0.11 mg/5 h. This urinary total protein excretion was significantly (F[8,45] =24.41, P < 0.001) increased to 3.10 ± 0.21 mg/5 h in zinc disc implanted animals [Table 2]. Treatment of animals with cystone (500 mg/kg), higher dose of CAM (400 mg/kg), and all three doses of MAM (100, 200, and 400 mg/kg) caused significant decrease in the urinary total protein excretion as compared to zinc disc implanted group [Tables 3 and 4].
Effect on serum creatinine, uric acid, and blood urea nitrogen levels
The mean serum creatinine level of sham-operated animals was 0.71 ± 0.02 mg/dl, which was significantly (F[8,45] =114.72, P < 0.001) increased to 1.88 ± 0.06 mg/dl in the zinc disc implanted animals [Table 2]. This increased serum creatinine level was significantly reduced by the treatment with cystone (500 mg/kg), higher dose of CAM (400 mg/kg), and all three doses of MAM (100, 200, and 400 mg/kg) in a dose-dependent manner [Tables 3 and 4].
Zinc disc implantation caused significant (F[8,45] =115.60, P < 0.001) increase in the serum uric acid level from 1.24 ± 0.04 mg/dl to 3.29 ± 0.10 mg/dl [Table 2]. This increased serum uric acid level was significantly reduced by the treatment with cystone (500 mg/kg), higher dose of CAM (400 mg/kg) and all three doses of MAM (100, 200, and 400 mg/kg) in dose-dependent manner [Tables 3 and 4].
The mean serum BUN level of sham-operated animals was 37.95 ± 0.74 mg/dl. This serum BUN level was significantly (F[8,45] =18.05, P < 0.001) increased to 51.13 ± 1.36 mg/dl in the zinc disc implanted animals [Table 2]. Treatment of animals with cystone, CAM, and MAM caused a significant decrease in the serum BUN level at a dose of cystone-500 mg/kg, CAM-400 mg/kg, MAM-100 mg/kg, MAM-200 mg/kg, and MAM-400 mg/kg, as compared to zinc disc implanted group [Tables 3 and 4].
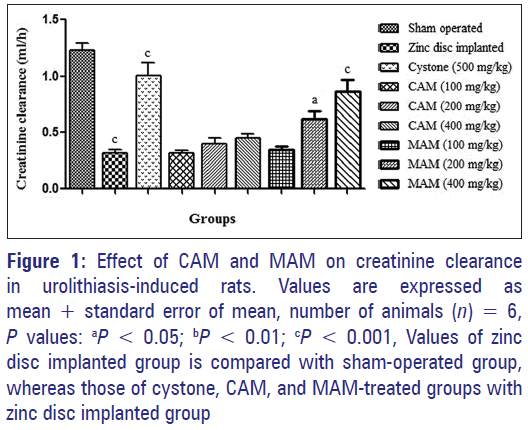
Effect on creatinine clearance
The mean creatinine clearance, i.e., glomerular filtration rate (GFR), of sham-operated animals was 1.23 ± 0.06 ml/h. This creatinine clearance was significantly (F[8,45] =26.55, P < 0.001) decreased to 0.32 ± 0.03 ml/h in the zinc disc implanted animals. Treatment of animals with cystone (500 mg/kg), intermediate and higher doses of MAM (200 and 400 mg/kg) caused a significant increase in the creatinine clearance as compared to zinc disc implanted group [Figure 1].
Figure 1: Effect of CAM and MAM on creatinine clearance in urolithiasis-induced rats. Values are expressed as mean + standard error of mean, number of animals (n) = 6, P values: aP < 0.05; bP < 0.01; cP < 0.001, Values of zinc disc implanted group is compared with sham-operated group, whereas those of cystone, CAM, and MAM-treated groups with zinc disc implanted group
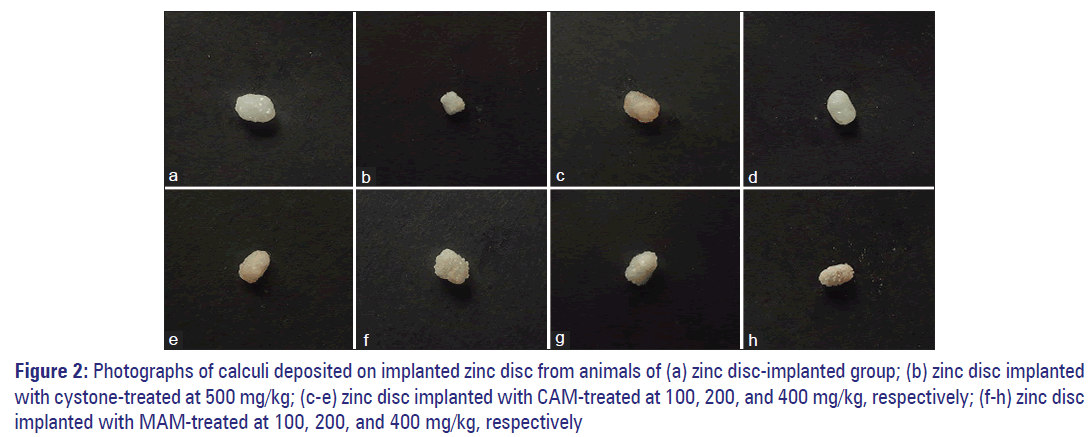
Effect on weight and dimensions of calculi
Zinc disc implantation caused deposition of calculi around the implanted disc, thereby significantly increased the weight (F[8,45] =91.43, P < 0.001), diameter (F[8,45] =70.05, P < 0.001) and thickness (F[8,45] =111.82, P < 0.001) of the implanted disc [Figure 2]. The mean weight of deposited calculi in zinc disc implanted animals was 78.32 ± 5.65 mg [Table 2]. Animals treated with cystone (500 mg/kg), higher dose of CAM (400 mg/kg), and all doses of MAM (100, 200, and 400 mg/kg) produced a significant decrease in the weight of calculi as compared to zinc disc implanted group. This reduced calculi deposition was also confirmed from the significant decrease in the diameter and thickness of the calculi [Tables 3 and 4].
Figure 2: Photographs of calculi deposited on implanted zinc disc from animals of (a) zinc disc-implanted group; (b) zinc disc implanted with cystone-treated at 500 mg/kg; (c-e) zinc disc implanted with CAM-treated at 100, 200, and 400 mg/kg, respectively; (f-h) zinc disc implanted with MAM-treated at 100, 200, and 400 mg/kg, respectively
Discussion
Urolithiasis is the third prevalent disorder of the urinary system. It may cause serious medical consequences such as extreme obstruction, hydronephrosis, infection, and hemorrhage in the urinary tract system. [20] The surgical operation, lithotripsy, and local calculus disruption using high-power laser are commonly used techniques to remove the calculi. However, these procedures are associated with the risk of acute renal injury leading to decrease in renal function. Moreover, an increase in stone recurrence is also observed. [21] All these facts indicate the need to develop a suitable alternative therapy for the treatment of urolithiasis. In Indian indigenous system of medicine, several plants including A. moschatus are claimed to be useful for the renal calculi. [22] Therefore, we have evaluated the antiurolithiatic potential A. moschatus in rats.
Renal calculi can be experimentally induced by either administration of various chemicals such as sodium oxalate or surgical implantation of foreign material like zinc disc in the urinary bladder of rats. The chemically-induced calculi model associated with high incidence of nephrotoxicity, metabolic acidosis, and occurrence of calculi in the renal cortex that is situation opposite to that found in human urolithiasis. [23] However, zinc disc model induces renal calculi with minimal renal damage and mimic the etiology of urinary stone formation in humans. [24] Therefore, zinc disc implantation-induced urolithiasis model was selected to induce urolithiasis in rats. In this model, the implanted zinc disc act as a nidus that subsequently leads to deposition of a urolith composed of magnesium ammonium phosphate around the disc. [25]
Consistent with earlier reports, urine output was decreased in disc implanted group. [26] This suggests obstruction of urinary bladder due to the formation of urinary calculi. In this study, the CAM and MAM treatment increased urine output, and this may be due to prevention of stone formation or their direct diuretic effect which reduced supersaturation of urine with precipitating substances and thereby stone formation.
As reported in some previous reports, a significant increase in the urinary total protein excretion was observed in disc implanted animals. [27] Supersaturation of urine with precipitating substances results in precipitation of crystal initiation particle which when trapped acts as a nidus leading to subsequent crystal growth. This is associated with proteinuria that reflects proximal tubular dysfunction. [28] Treatment of CAM and MAM showed a significant reduction in the protein excretion and thus might have prevented the nidus formation of crystal formation. The CAM and MAM produced the initial effect of enhancing urinary output, further shown a reduction in protein excretion which is an indirect indication of either recovery of urinary dysfunction or prevention of crystal-induced damage. This action of CAM and MAM is considered to the most useful outcome because CAM and MAM not provide only symptomatic action but also has its direct action at the membrane level.
Nucleation and subsequent growth of renal calculi are next step which involves lipid peroxidation and cause renal damage by reacting with polyunsaturated fatty acids in the cell membrane. [29] This renal damage is indicated by the elevated serum levels of creatinine, uric acid, and BUN, which are markers of glomerular and tubular damage. In this study, there was a significant increase in the serum levels of creatinine, uric acid, and BUN of disc implanted animals, whereas treatment with CAM and MAM showed to prevent the elevation of serum levels of these markers. This indicates that CAM and MAM act by inhibiting the lipid peroxidation and thereby reduces the extent of tubular dysfunction.
In addition, a significant decrease in the GFR was also observed in disc implanted animals. This indicates that the animals were suffering from compromised renal functions due to increased size of urinary calculi which is in accordance with earlier published reports. [27] The MAM treatment showed to increase the GFR, which may be due to its collective effects of diuretic action and control on the growth of calculi. These observed actions at every step from enhanced urine output to improved GFR may be due to effects of various phytoconstituents that interact to give synergistic effect with the putative mechanism of action.
Consistent with previous reports, implantation of zinc disc in the bladder of rats caused accumulation of calculi around the disc. [27] The weight of disc is a direct method to evaluate antiurolithiatic activity wherein CAM and MAM have shown significant reduction which is in accordance with the other results of this study further supporting that CAM and MAM have antiurolithiatic potential. This antiurolithiatic potential is observed at all doses (100, 200, and 400 mg/kg) of MAM, whereas only higher dose (400 mg/kg) of CAM showed significant potential this study.
An earlier study reported a potent diuretic, antioxidant, and free-radical scavenging capacity of A. moschatus extracts in animals. Therefore, A. moschatus extracts may prevent calculi formation by diuretic, antioxidant, and free-radical scavenging constituents of the plant. [3-5]
Conclusion
The study concluded that the administration of A. moschatus seed extracts showed significant antiurolithiatic activity. Methanolic extract was found to be more effective than the chloroform extract of the plant. The exact mechanism of action is not established but may be mediated collectively through diuretic, antioxidant, and free-radical scavenging properties of the plant. However, further phytochemical exploration is essential to establish the exact mechanism of action.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Rao YR, Jena KS, Sahoo D, Rout PK, Ali S. Safety evaluation of Ambrette (Abelmoschus moschatus Linn) seed oil. J Am Oil Chem Soc 2005;82:749-52.
- Khare CP, editors. Encyclopedia of Indian Medicinal Plants: Rational Western Therapy, Ayurvedic and Other Traditional Usage, Botany. Berlin, Heidelberg (NY): Springer-Verlag Publisher; 2004.
- Mantena KR, Soni D. Diuretic activity of extract of Abelmoschus moschatus L. Asian Pac J Trop Biomed 2012;1:1-3.
- Christina AJ, Muthumani P. Phytochemical investigation and diuretic activity of Abelmoschus moschatus Medikus. Int J Pharm Chem Sci 2012;1:1311-4.
- Gul MZ, Bhakshu LM, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement Altern Med 2011;11:64.
- Maheshwari P, Kumar A. Antimicrobial activity of Abelmoschus moschatus leaf extracts. Curr Trends Biotechnol Pharm 2009;3:260-6.
- Dokka MK, Davuluri SP. Antimicrobial activity of a trypsin inhibitor from the seeds of Abelmoschus moschatus L. Int J Curr Microbiol Appl Sci 2014;3:184-99.
- Christina AJ, Muthumani P. Phytochemical investigation and antilithiatic activity of Abelmoschus moschatus medikus. Int J Pharm Pharm Sci 2013;5:108-13.
- Singh AK, Singh S, Chandel HS. Evaluation of hepatoprotective activity of Abelmoschus moschatus seed in paracetamol induced hepatotoxicity on rat. IOSR J Pharm 2012;2:43-50.
- Nandhini S, Vadivu R, Jayshree N. Memory strengthening activity on seeds of Abelmoschus moschatus. Int J Res Pharm Chem 2014;4:346-50.
- Liu IM, Liou SS, Lan TW, Hsu FL, Cheng JT. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med 2005;71:617-21.
- Dokka MK, Konala G, Davuluri SP. Hemagglutinating activity of trypsin inhibitors from the seeds of Abelmoschus moschatus L. Int J Adv Res 2014;2:892-903.
- Rival D, Bonnet S, Sohm B, Perrier E. A Hibiscus Abelmoschus seed extract as a protective active ingredient to favour FGF-2 activity in skin. Int J Cosmet Sci 2009;31:419-26.
- Sheik HS, Vedhaiyan N, Singaravel S. Evaluation of Abelmoschus moschatus seed extract in psychiatric and neurological disorders. Int J Basic Clin Pharmacol 2014;3:845-53.
- Khandelwal KR, editors. Practical Pharmacognosy. Pune, India: Nirali Prakashan; 2003.
- Rajesh P, Latha S, Selvamani P, Kannan VR. Phytochemical screening and toxicity studies on the leaves of Capparis sepiaria Linn. (Capparidaceae). J Basic Clin Pharm 2009;1:41-6.
- Sheik HS, Vedhaiyan N, Singaravel S. Acute and sub-acute toxicity studies of some indigenous medicinal plants. Int J Pharm Phytopharmacological Res 2013;3:166-9.
- Araujo Viel T, Diogo Domingos C, da Silva Monteiro AP, Riggio Lima-Landman MT, Lapa AJ, Souccar C. Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J Ethnopharmacol 1999;66:193-8.
- Taur DJ, Patil RY. Antihistaminic activity of Clitoria ternatea L. roots. J Basic Clin Pharm 2010;2:41-4.
- Hadjzadeh MA, Khoei A, Hadjzadeh Z, Parizady M. Ethanolic extract of Nigella sativa L. seeds on ethylene glycol-induced kidney calculi in rats. Urol J 2007;4:86-90.
- Begun FP, Knoll CE, Gottlieb M, Lawson RK. Chronic effects of focused electrohydraulic shock waves on renal function and hypertension. J Urol 1991;145:635-9.
- Warrier PK, Nambiar VP, Ramankutty C, editors. Indian Medicinal Plants – A Compendium of 500 Species. Chennai, India: Orient Longman Publishers; 1994.
- Hwisa NT, Assaleh FH, Gindi S, Melad FE, Chandu BR, Katakam P. A study. A study on antiurolithiatic activity of Melia Azadirachta L. aqueous extract in rats. Am J Pharmacol Sci 2014;2:27-31.
- Khan SR. Animal models of kidney stone formation: An analysis. World J Urol 1997;15:236-43.
- Yamaguchi S, Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS. Study of Study of a rat model for calcium oxalate crystal formation without severe renal damage in selected conditions. Int J Urol 2005;12:290-8.
- Vyas N, Argal A. Antiurolithiatic activity of extract and oleanolic acid isolated from the roots of Lantana camara on zinc disc implantation induced urolithiasis. ISRN Pharmacol 2013;2013:951795.
- Singh PK, Patil CR, Harlalka GV, Gaud NP. Zinc disc implantation model of urinary bladder calculi and humane endpoints. Lab Anim 2010;44:226-30.
- Selvam R, Kalaiselvi P, Govindaraj A, Bala Murugan V, Sathish Kumar AS. Effect of A. lanata leaf extract and Vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res 2001;43:89-93.
- Karadi RV, Gadge NB, Alagawadi KR, Savadi RV. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 2006;105:306-11.