Antimicrobial resistance pattern in a tertiary care hospital: An observational study
- *Corresponding Author:
- Dr. Revathy Saravanan
Department of Pharmacology, Sri Venkateshwaraa Medical College, Hospital and Research Centre, Ariyur, Pondicherry, India.
E-mail: dr_rsmail@yahoo.co.in
Abstract
Context: The number of organisms developing resistance to commonly used antibiotics is increasing among the various generations. The exact national scenario of antimicrobial resistance (AMR) is not known in India owing to the absence of a central monitoring agency. Aims: The aim of this study is to identify the group of organisms developing resistance, to know the classes of drugs against, which resistance has emerged and to assess the possible factors that can favor the development of AMR so that antibiotic policy can be formulated for the proper and effective use of antibiotics. Settings and Design: An observational study was conducted for a period of 1 year from August 2011 to July 2012 in a tertiary care hospital in Pondicherry. Subjects and Methods: Data regarding culture and sensitivity of the organisms isolated from different sources such as urine, blood, wound swab/pus, stool, sputum and tracheal aspirations were collected from the records of the Microbiology Department. Sample processing, identiô€ÂÂÂÂÂÂication of organisms to the genus and/or species level and antimicrobial sensitivity were carried out as per the Clinical and Laboratory Standards Institute guidelines on the 999 samples received. Results: Out of 999 samples, 125 (12.5%) showed signiô€ÂÂÂÂÂÂicant growth of organisms exhibiting resistance to either single or multiple drugs. Out of 84 (67.2%) in-patients and 41 (32.8%) out-patient samples, Escherichia was the most common organism isolated with a total of 41 (32.8%), followed by Methicillin sensitive Staphylococcus aureus, 26 (20.8%), Klebsiella 25 (20%), Methicillin resistant Staphylococcus aureus 17 (13.6%), Pseudomonas 10 (8%), Proteus 2 (1.6%), 1 (0.8%) each of Citrobacter and Enterococci. Maximum resistance was observed with commonly used ô€ÂÂÂÂÂÂirst line antimicrobials such as co-trimoxazole, ampicillin, amoxicillin, amoxyclav, ô€ÂÂÂÂÂÂluoroquinolones, third generation cephalosporins and nalidixic acid. Least resistant or highly sensitive were amikacin, nitrofurantoin, gentamycin and doxycycline among the gram-negative bacteria. Macrolides, clindamycin, gentamycin, nitrofurantoin, vancomycin were the most sensitive antimicrobials against the gram-positive bacteria. Lack of knowledge on the consequences of inappropriate use of antibiotics was exhibited by 63% of subjects in our study. Conclusions: AMR was more with hospital acquired organisms and against commonly used antibiotics that are available since long period. Variation of resistance and sensitivity pattern with time and geographical location is identiô€ÂÂÂÂÂÂied. Periodic AMR monitoring and rotation of antibiotics are suggested to restrict further emergence of resistance.
Keywords
Antibiotics, antimicrobial resistance, observational study
Introduction
Antimicrobial resistance (AMR), a growing public health concern where the microorganism is able to survive exposure to antibiotic treatment. [1] This is evident from the first report of vancomycin resistant Staphylococcus aureus (VRSA) from the US in 2002, Brazil in 2005, Jordan and India in 2006. Similarly, resistance was reported in the late 1980s, with vancomycin resistant Enterococci. Controlling infections is going to be a tough job in developing countries like India where infectious diseases still hold high morbidity and mortality. [1,2] Methicillin resistant Staphylococcus aureus (MRSA) identified in 1990 soon after the introduction of pencillinase resistant penicillins, started as a single clonal mutation and resulted in community acquired MRSA owing to diversification of clones. [1]
Several intrinsic factors such as point mutation, gene amplification and extrinsic factors like horizontal transfer of resistant gene between bacteria within and across species by transposons, integrins or plasmids have been postulated for the development of resistance, which cannot be reduced once developed even by restricting the antibiotic usage. Social factors such as demographic changes, deficient hygienic practices and overcrowding have been enumerated for the emergence of AMR and this is supported by the multidrug resistant (MDR) Escherichia coli that has been isolated in carriers and in water samples by a study carried out in rural Tamil Nadu. [1,3] Inappropriate and irrational uses of antibiotics in humans and animals for therapeutic and non-therapeutic use (as growth promoters) have been focused as main causes for the emergence of hospital and community acquired resistant infections by World Health Organization (WHO). This was also evidenced by the presence of MDR E. coli in cow dung and drinking water in a study conducted in Odisha. [4,5]
Geographical variation in sensitivity is also noted by studies conducted in North India, which showed vibrio cholera being resistant to furazolidone, co-trimoxazole and nalidixic acid but sensitive to tetracycline around Delhi, but resistance was noted against tetracycline in Bangladesh. [1] Extended spectrum β-lactamase (ESBL) was first proposed in 1987. [6] Three clinically available β-lactamase inhibitor that can be combined with β-lactams to reduce hydrolysis, are effective against class-A β-lactamases only and not against class B, C, D lactamases. Other class B-carbapenamase inhibitors, which can be effective against carbapenamase producing organisms is under study. [6] The increase in susceptibility to antibiotics by previously resistant gram negative organisms by following antibiotic policy and by antibiotic rotation has been demonstrated in a study done on ventilator associated pneumonia among intensive care units (ICU) patients who were started on antibiotics empirically by Didier Gruson et al. in France. [7]
The national scenario of AMR is not known in India due to the absence of central monitoring agency. In view of this fact and due to the geographical and time based variations in antibiotic resistance and sensitivity that have been reported by many studies this study was undertaken with the following objectives in a tertiary care rural hospital in the coastal region of Tamil Nadu:
1. To identify the group of organisms developing resistance
2. To identify the classes of drugs against, which resistance has emerged
3. To assess the possible factors that can favor the development of AMR so that antibiotic policy can be formulated for the proper and effective use of antibiotics.
Subjects and Methods
An observational study was conducted for a period of 1 year from August 2011 to July 2012 in a tertiary care hospital in Pondicherry. Approval from the Institutional Research and Ethical committee was obtained prior to the commencement of the study. The data regarding culture and sensitivity of the organisms isolated from different sources such as urine, blood, wound swab/pus, stool and other sources such as sputum and tracheal aspirations were collected from the records of the Microbiology Department, which included both out-patients (OP) and in-patients (IP). A total of 999 non-repetitive samples during the study period were included. Sample processing, identification of organisms to the genus/ species level and antimicrobial sensitivity (HiMedia, Micro Express) were carried out as per the Clinical and Laboratory Standards Institute guidelines.
Antibiotics tested for sensitivity against gram negative bacteria were ampicillin, amoxyclav, gentamycin, amikacin, ciprofloxacin, co-trimoxazole, cefotaxime, ceftriaxone, ceftazidime, norfloxacin, nalidixic acid, nitrofurantoin, doxycycline, Antibiotic profile for gram-positive bacteria was gentamycin, ciprofloxacin, oxacillin, clindamycin, erythromycin, co-trimoxazole, doxycycline and vancomycin. Organisms resistant to more than one drug were considered as MDR. After getting an informed consent from the subjects, they were requested to answer a simply formulated questionnaire to the fullest of their knowledge to elicit their prior antibiotic history. Other relevant data was obtained from the patient’s case sheet. All data were tabulated and analyzed. Descriptive statistics was used for analysis and the results were expressed.
Results
The total number of samples sent to our microbiology laboratory from out- and in-patients of the various departments for culture and sensitivity during the period from August 2011 to July 2012 was 999. Out of this, 244 samples were from patients attending the OP department and 755 were IP).
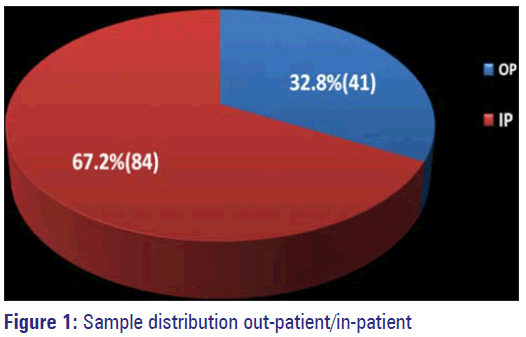
Out of 999 samples, 125 (12.5%) showed significant growth of organisms and exhibited resistance to either single or multiple drugs, these were 84 (67.2%) from IP and 41 (32.8%) from OP. Remaining samples either had no organisms grown or had insignificant growth (urine samples). Among the IP samples, 42 were from Pus, 35 were urine samples, one each from blood, sputum, tracheal aspirate and four from stool samples. Out of 41 OP samples 16 were Pus, 23 Urine, one blood and one stool sample (Figure 1,Table 1).
| Type of sample | IP | OP | Total |
|---|---|---|---|
| Pus/wound swab | 42 | 16 | 58 |
| Urine | 35 | 23 | 58 |
| Blood | 1 | 1 | 2 |
| Sputum | 1 | 1 | |
| Tracheal aspirate | 1 | - | 1 |
| Stool | 4 | 1 | 5 |
| Total | 84 | 41 | 125 |
IP: In-patient, OP: Out-patient
Table 1: Distribution of samples among IP/OP
Organisms isolated from various samples were E. coli, Klebsiella and Pseudomonas, Methicillin sensitive Staphylococcus aureus (MSSA), MRSA, Proteus, Enterococci, Acinetobacter and Citrobacter.
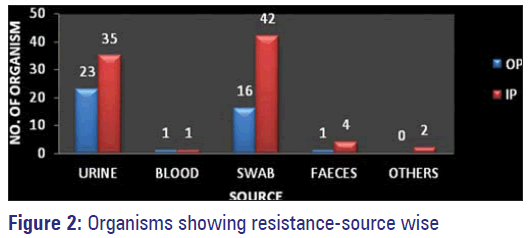
E. coli was the most common organism isolated with a total of 41 (32.8%). Out of the 41 samples, 26 were IP samples and 15 were from OP. 34 E. coli isolates were from urine, two from pus/wound swab and five from stool samples from pediatrics below the age of 3 years. A total of 26 (20.8%) MSSA were isolated, 20 from wound/pus swabs and six from urine samples. Out of the 26 (21%) MSSA isolates, 19 were from IP and seven were from OP samples. Klebsiella isolates were a total of 25 (20%). They were isolated from various samples such as urine (13), pus/wound swabs (8), blood (2), sputum (1) and tracheal aspirate (1). 17 were isolated from IP and eight from OP. The total number of MRSA isolates were 17 (13.6%), out of which 11 were from IP and six was from OP. 16 MRSA were isolated from pus/wound swabs and one from the urine sample. 10 (8%) isolates of Pseudomonas were isolated from wound/pus swabs (9) and urine samples (one), nine were IP and one was from a OP sample. Proteus, (2/1.6%) was isolated one each from urine and pus/wound swab and the same were from IP and OP respectively. 2 (1.6%) Acinetobacter isolates were from pus/wound swabs and one each was from IP and OP. 1 (0.8%) each Citrobacter and Enterococci were isolated from the urine sample and both were colonized in OP [Tables 2 and 3, Figure 2].
| Organism | Pus | Urine | Stool | Blood | Sputum | Tracheal aspirate | Total |
|---|---|---|---|---|---|---|---|
| E. coli | 2 | 34 | 5 | - | - | - | 41 |
| MSSA | 20 | 6 | - | - | - | - | 26 |
| Klebsiella | 8 | 13 | - | 2 | 1 | 1 | 25 |
| MRSA | 16 | 1 | - | - | - | - | 17 |
| Pseudomonas | 9 | 1 | - | - | - | - | 10 |
| Proteus | 1 | 1 | - | - | - | - | 2 |
| Acinetobacter | 2 | - | - | - | - | - | 2 |
| Citrobacter | 1 | - | - | - | - | 1 | |
| Enterococci | 1 | - | - | - | - | 1 | |
| Total | 58 | 58 | 5 | 2 | 1 | 1 | 125 |
E. coli: Escherichia coli, MSSA: Methicillin sensitive Staphylococcus aureus, MRSA: Methicillin resistant Staphylococcus aureus
Table 2: Distribution of isolated organisms among various samples
| Organism | IP | OP | Total |
|---|---|---|---|
| E. coli | 26 | 15 | 41 |
| SSA | 19 | 7 | 26 |
| Klebsiella | 17 | 8 | 25 |
| MRSA | 11 | 6 | 17 |
| Pseudomonas | 9 | 1 | 10 |
| Proteus | 1 | 1 | 2 |
| Acinetobacter | 1 | 1 | 2 |
| Citrobacter | 1 | 1 | |
| Enterococci | 1 | 1 | |
| Total | 84 | 41 | 125 |
OP: Outpatient, IP: Inpatient, E. coli: Escherichia coli, MSSA: Methicillin sensitive Staphylococcus aureus, MRSA: Methicillin resistant Staphylococcus aureus
Table 3: Distribution of organisms isolated from IP and OP
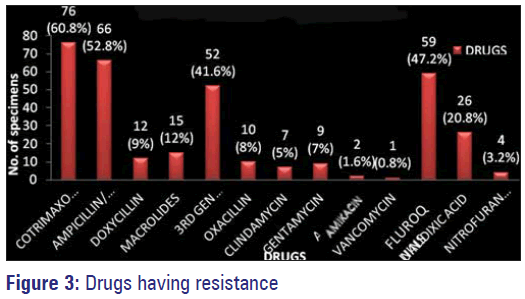
Among the commonly used antimicrobials against gram negative and gram positive organisms in our study maximum resistance was observed with co-trimoxazole followed by ampicillin, amoxicillin, amoxyclav, fluoroquinolones, third generation Cephalosporins and nalidixic acid. Least resistance or the highly sensitive antimicrobials to these organisms isolated was observed with amikacin, nitrofurantoin, gentamycin, clindamycin, doxycycline, macrolides and vancomycin [Figure 3].
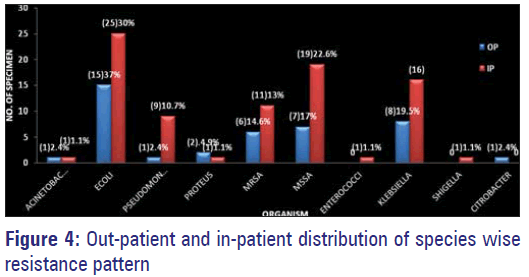
Maximum resistance to multiple antimicrobials by organisms were observed among those isolated from IP samples, with E. coli topping the list followed by MSSA, Klebsiella, MRSA and Pseudomonas [Figure 4].
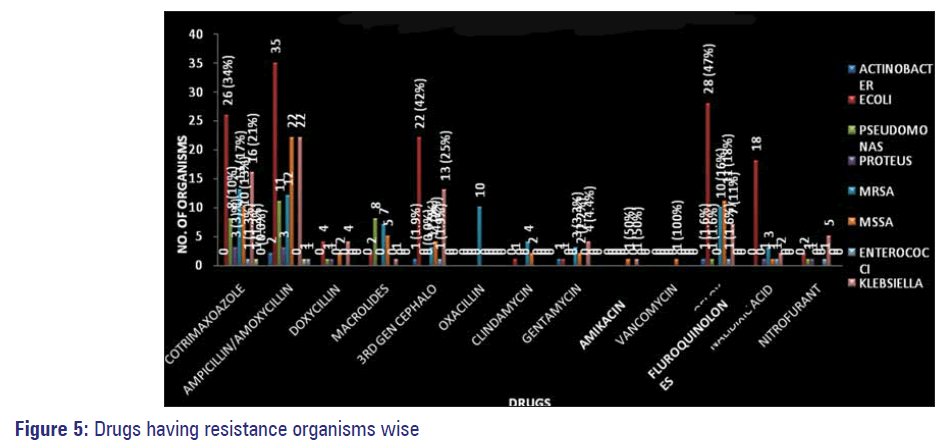
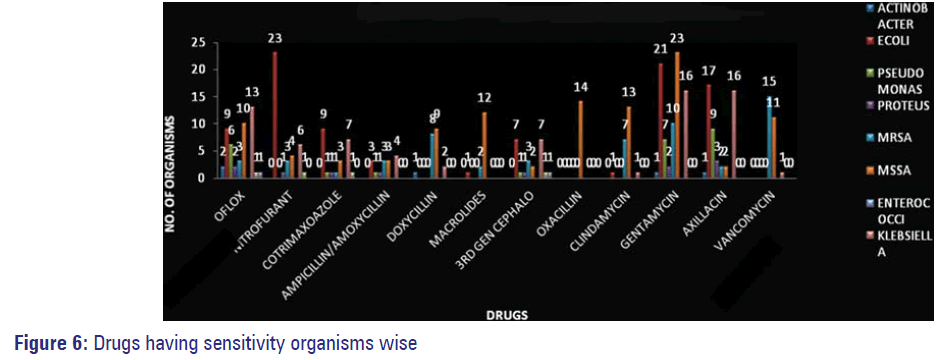
Organisms isolated from pus/wound swabs were the most resistant ones followed by those isolated from urine samples Figure 5. Sensitivity of isolated resistant organisms to various antimicrobials is depicted in Figure 6.
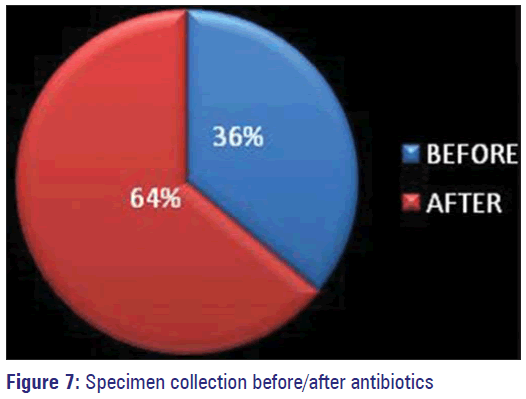
Antibiotics were started empirically before sending the specimens for culture sensitivity in 64% of patients, which includes both IP and OP.
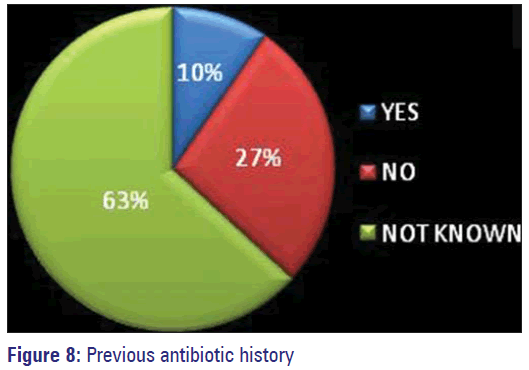
In 36% of patients specimens were collected before starting antimicrobials. Those for whom change of antibiotics was prescribed based on the reports, many of them had good outcome. Previous antimicrobial exposure history could not be elicited from 63% of study subjects owing to their unawareness. The percentage of patients who said they have not consumed any antibiotic was 20% and 10% accepted having used antimicrobials before.
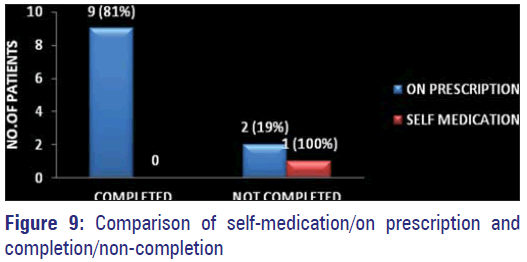
Out of 12 patients (10%) who gave history of prior exposure to antibiotics 11 have stated to have used only on prescription by physicians and nine of them had completed the prescribed regimen leaving only two patients who have discontinued on their own.
Discussion
In our observational study conducted over a period of 1 year, the number of samples received from hospitalized patients was almost double of that from the community. The gram-negative and few gram-positive (MRSA and MSSA) organisms isolated from hospitalized patients exhibited more resistance when compared with outpatients. This pattern is supported by the WHO community surveillance study report on pathogenic organisms, which suggest that more number of resistant organisms were observed among hospitalized patients than community acquired organisms. [8] E. coli was found to be the most common and highly resistant organism in our study (32.8%), which was isolated mostly from urine, stool and pus samples. Similarly, high percentage of ESBL producing E. coli has been reported with 60% in a study done at a tertiary care hospital in Chennai. [9] More number of MDR E. coli was noted in hospitalized patients than in OP in our study, which is consistent with reports from many other similar studies. [8,10,11] However, less incidence of hospital acquired MDR E. coli with 8% has been reported in a study conducted at AIIMS, Delhi in 2001. [12]
Highest resistance by E. coli was noted against ampicillin/ amoxicillin followed by fluoroquinolones, co-trimoxazole, third generation cephalosporins and nalidixic acid in our study. This pattern of resistance has been shown by many studies. [8,9,10,12] Resistance to nitrofurantoin was least, which is in accordance with the report from community based pilot study by WHO in Vellore, Tamil Nadu on both the phases between August 2003 and December 2005. Least resistance was observed in our study against gentamycin, amikacin, which is similar to the community based surveillance study done by WHO. [8] Gentamycin sensitivity was slightly higher compared with amikacin in our study (21 vs. 17 respectively) in contrast to higher sensitivity of 68% to amikacin and 9% to gentamycin in a prospective study performed in a tertiary care hospital in Pondicherry during 2011. [10] This supports the possibility of changing sensitivity pattern with time difference. [1]
MSSA was the next common organism isolated (21%), but little higher incidence of 51.63% has been reported by another tertiary care hospital study done in Pondicherry. [13,14] Resistance was observed mainly to ampicillin/amoxicillin than to the fluroquinolones in this study with minimal resistance to other drugs such as macrolides, co-trimoxazole, clindamycin and doxycycline. Gentamycin has been found to be more effective against MSSA like in a similar study conducted by a hospital in Pondicherry. [14] MSSA having 100% sensitivity to vancomycin has been reported by another tertiary hospital in Pondicherry in the same year 2012, but only 50% of MSSA showed sensitivity to vancomycin in our study. [14]
High prevalence of MRSA, especially in hospitalized post-surgical and trauma patients has been observed by many studies. [15-18] Hospital acquired MRSA has been proved to be more virulent and MDR than community acquired by many previously carried out studies also. [15,17] MRSA showed high resistance to co-trimoxazole and ampicillin/amoxicillin followed by fluoroquinolones in our study. Least resistance was found to be against macrolides, clindamycin and gentamycin. Vancomycin and amikacin were found to be 100% effective on MRSA. Similar resistance and sensitivity pattern has been demonstrated by many other studies also. [12,14,16,18-20] and 100% sensitivity has been reported to linezolid by yet another study. [18] However, least resistance of 4.8% has been reported in a study done among fishermen along the coastal area of Tamil Nadu in 2012. [15]
The emergence of vancomycin intermediate resistant Staphylococcus aureus (VISA) has been reported from Jawaharlal Institute of Postgraduate Medical Education and Research, South India during 2004-2006. The high incidence of MRSA has been reported to be on the rise by many studies as 43% in Davengare, 55% by Banaras Hindu university, 39% by AIIMS, 31% by a Chennai hospital, but low incidence of 29% in Nepal in 1990,18% from Hubli and low incidence in North-eastern part of India has also been reported. [12,18,21,22] Continuous monitoring of minimum inhibitory concentration MIC levels to still sensitive drugs like vancomycin and restriction of its use can be suggested due to the possibility of MRSA becoming fully resistant to vancomycin, which has remained the best alternative to control infections with MRSA so far.
The occurrence of MDR Pseudomonas was observed in 10 out of 125 samples in our study (9-IP and 1-OP) confirming it to be the most common Hospital acquired infection as evidenced by many studies conducted at different parts of India in addition to studies done in AIIMS, Delhi and from ICUs of seven different hospitals in Goa. [11,23,24] Pus/swab were the major source from which this organism was isolated similar to many other reports. [13] Sensitivity was observed more to amikacin than to fluoroquinolones in our study [Figure 6]. Higher resistance to ampicillin/amoxicillin and equal resistance to co-trimoxazole were noted in our study. Low sensitivity was observed against third generation cephalosporins in our study, which is similar to the reports given by Ganguly et al. of India National working group-India in its situation analysis performed at Delhi, Vellore and Mumbai in 2011. [12]
In our study, Klebsiella, one of the main bacterial pathogen seen in complicated influenza virus infection of common upper respiratory infections such as sore throat, pharyngitis etc., and also a hospital acquired infection as per India report on Global resistance, was observed to be resistant to drugs such as ampicillin/amoxicillin, co-trimoxazole, third generation cephalosporins, fluoroquinolones, nitrofurantoin [Figure 5]. Least resistance was exhibited to gentamycin, amikacin and nalidixic acid mimicking the results of many studies. [10,12,13,24-26] Proteus showed resistance to co-trimoxazole, ampicillin/ amoxicillin, doxycycline and nitrofurantoin and sensitivity to amikacin, gentamycin and fluoroquinolones, compared to 100% sensitivity reported by ShashiChopra et al. among ICU patients in his study. [26]
Enterococci was isolated from one urine sample and showed sensitivity to amikacin, gentamycin, doxycycline and co-trimoxazole. Incidence of 28% isolation of Enterococci from blood culture of ICU patients at Punjab Institute of Medical Sciences, Jalandhar has been reported with similar sensitivity pattern. [26] Acinetobacter was isolated from pus/swab sample with 50% resistant to fluoroquinolones and gentamycin and 50% sensitivity to amikacin and co-trimoxazole in our study. Similar resistance and sensitivity pattern has been demonstrated in a study at St. John’s Hospital Bangalore in March 2003 to March 2004 [12]
Citrobacter was isolated from one urine sample, being resistant to nitrofurantoin, ampicillin and amoxicillin but sensitive to third generation cephalosporins, co-trimoxazole and fluoroquinolones in the study done by us. Among the antimicrobials used in our study for culture sensitivity highest resistance was demonstrated against co-trimoxazole followed by ampicillin/amoxicillin, which might be due to the easy availability over-the-counter and easy oral self-administration. Quinolones and third generation cephalosporins were shown to be resistant in our study. Quinolones was reported to be the more effective and used most both in phase-1a and phase-1b of community based surveillance study performed in five locations by WHO between August 2003 and December 2005. [8,12] This increased use of Quinolones might have resulted in increased resistance to them, which could have pushed down the use of Quinolones and increased that of third generation cephalosporins, which was observed by the author in the surveillance study of antibiotic use in Pondicherry done in 2012. [27] Increasing incidence of resistance to third generation cephalosporins also has been noted in our study probably due to the increasing emergence of ESBL producing organisms following increased usage. Less resistance was noted against drugs such as gentamycin and amikacin. Restricted use of these drugs because of the cost and the need for assistance for parentaral administration may be the contributing factor for remaining sensitive still.
Resistance can be developed against them also by synthesis of inactivating enzymes by the organisms if unnecessary use is not restricted. Vancomycin was found to be 100% effective against most of the organisms by us also similar to many studies. This being the best alternative choice for MRSA, which is on the increase is to be handled carefully only against organisms that are sensitive only to them as the emergence of VRE and VRSA have been already reported by a study done in Pondicherry. [22] Empirical use of antibiotics in the community and in hospitalized patients has been highlighted in some studies. [11,23,27] Such initial empirical use of antibiotics without waiting for the sensitivity reports has been observed in our study also among 64% of the study population [Figure 7]. Inappropriate use of antibiotics being an important contributing factor for the development of resistance has already been established. However, in a study among primary school children in rural Tamil Nadu, recent history of medication use was not identified as a significant risk factor for the emergence of Multi Drug Resistant organisms. [3]
The lack of knowledge about the consequences of inappropriate use of antibiotics by the consumers, mainly the patients has been identified as an important cause for the emergence of A.M.R. [12,27] This ignorance was also exhibited by 63% of our study population [Figure 8]. Only 12 out of 125 patients were able to give details about the prior antibiotic history and nine persons have stated to have used on prescription and they have completed the course of treatment as prescribed [Figure 9]. Hospital acquired organisms were proved to be more resistant than the community acquired by many studies. [11,17] Nasal carriage of MDR MRSA even without prior antibiotic exposure has been identified in a study conducted among school going children belonging to different areas of Bangalore city. [16] Development of resistance was more by gram negative organisms than by gram-positive ones. Increasing occurrence of MDR MRSA among positive organisms has posed a great threat. Hence, restriction of unnecessary use of antibiotics and the emergence of AMR globally has to be implied effectively and quickly to prevent the occurrence of the post-antibiotic era making control of infections a very difficult task. Strengthening of Pandemic Surveillance for AMR by means of training courses and distribution of WHONET - a surveillance supporting computer program has been planned by International Health Regulation 2005. [2]
Conclusion
In the 1 year observational study performed by researchers, MDR organisms were isolated in 125 samples, which were mostly from pus/swab mainly from post-operative hospitalized patients. Gram-negative organisms like E. coli, Pseudomonas Aeroginosa, Klebsiella pneumonia and Proteus were found to be the resistant ones highest being with E. coli. Among the gram-positive organisms MSSA, MRSA and Enterococci were identified as the resistant organisms. All were resistant to more than 2-3 antibiotics and sensitivity also was identified against more than 2-3 antibiotics. AMR was more with hospital acquired ones than that from the community. Most commonly used antibiotics for a long period such as co-trimoxazole; ampicillin/amoxicillin, fluoroquinolones and third generation cephalosporins were found to be the primary drugs against, which resistance was exhibited by more than 2-3 organisms. Intermediate sensitivity was shown for doxycycline and macrolides.
The parentarally used drugs like amino glycosides (e.g. gentamycin and amikacin) and vancomycin have retained their sensitivity against hospital acquired infections like Pseudomonas, MRSA and Klebsiella. Geographical and time based variations in resistance and sensitivity pattern was clearly observed by us on comparing with previous reports. However, we were not able to correlate the emergence of AMR to prior antibiotic exposure owing to the ignorance expressed by the majority of the participants. The total number of samples in our study was comparatively small to other referred studies. Another limiting factor was that all isolated organisms were not exposed to all the antibiotics, which were chosen, based on the organism. The changing pattern of the resistant organisms could be identified in this 1 year single center study in spite of these restrictions. A better opinion can be obtained on analysis of culture sensitivity results from multiple centers. In addition to other policies like promoting rational use of antibiotics, enhancement of knowledge among the prescribers and in the community, periodic ecology based monitoring of AMR pattern taking into account the dosage, rhythm of antibiotics will definitely be of great help in formulating appropriate antibiotic policy. On strict follow-up of such formed guide lines based on such antibiotic policy will help in controlling infections, which is still stated as a factor for morbidity and mortality in developing countries like India. As return of sensitivity by previously resistant organisms has been documented by many reports antibiotic rotation can be thought of as another solution. The possibility of screening health-care workers who come in to contact with carriers can lead to hospital acquired infections and disinfecting them with appropriate antibiotic schedules can be considered to prevent the emergence of MDR nosocomial infections. However, cost-effectiveness of such strategy is to be analyzed..
References
- Raghunath D. Emerging antibiotic resistance in bacteria with special reference to India. J Biosci 2008;33:593-603.
- Gupta SK, Gupta P, Sharma P, Shrivastava AK, Soni SK. Emerging and re-emerging infectious diseases future challenges and Strategy. J Clin Diagn Res 2012;6:1095-100.
- Seidman JC, Anitha KP, Kanungo R, Bourgeois AL, Coles CL. Risk factors for antibiotic-resistant E. coli in children in a rural area. Epidemiol Infect 2009;137:879-88.
- Sahoo KC, Tamhankar AJ, Sahoo S, Sahu PS, Klintz SR, Lundborg CS. Geographical variation in antibiotic-resistant Escherichia coli isolates from stool, cow-dung and drinking water. Int J Environ Res Public Health 2012;9:746-59.
- Kulkarni SV, Narayan A, Indumathi VA, Tejas, Rao S, Kempegowda P. Salmonella Paratyphi A in India-Changing trends in presentation and antibiotic susceptibility. Asian J Med Sci 2011;2:14-7.
- Lee JH, Bae IK, Lee SH. New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med Res Rev 2012;32:216-32.
- Gruson D, Hilbert G, Vargas F, Valentino R, Bebear C, Allery A, et al. Rotation and restricted use of antibiotics in a medical intensive care unit. Impact on the incidence of ventilator-associated pneumonia caused by antibiotic-resistant gram-negative bacteria. Am J Respir Crit Care Med 2000;162:837-43.
- World Health Organization. Community-based surveillance of antimicrobial use and resistance in resource-constrained settings. Report on five pilot projects. WHO/EMP/MAR/2009.
- Narayanaswamy A, Mallika M. Prevalence and susceptibility of extended spectrum beta-lactamases in urinary isolates of Escherichia coli in a tertiary care hospital, Chennai-South India. Internet J Med Update 2011;6:39-43.
- Umadevi S, Kandhakumari G, Joseph NM, Kumar S, Easow JM, Stephen S, et al. Prevalence and antimicrobial susceptibility pattern of ESBL producing gram negative bacilli. J Clin Diagn Res 2011;5:236-9.
- Jamshidi M, Javadpour S, Eftekhari TE, Moradi N, Jomehpour F. Antimicrobial resistance pattern among intensive care unit patients. Afr J Microbiol Res 2009;3:590-4.
- Ganguly NK, Wattal C, Chandy SJ, Arora SK. Gupta U, Kotwani A, et al. National Working Group Situation analysis: Antibiotic use and resistance in India. Global Antibiotic Resistance Partnership-India. March, 2011.
- Peripi SB, Thadepalli VG, Khagga M, Tripuraribhatla PK, Bharadwaj DK. Profile of antibiotic consumption, sensitivity and resistance in an urban area of Andhra Pradesh, India. Singapore Med J 2012;53:268-72.
- Kumar S, Joseph NM, Easow JM. Prevalence and current antibiogram of staphylococci isolated from various clinical specimens in a tertiary care hospital in Pondicherry. Internet J Microbiol 2012;10:1937-8289.
- Mohankumar A, Tamil Selvi S. Antibiotic pattern of methicillin resistant Staphylococcus aureus isolated from chronic wound of fisherman community. Int J Microbiol Res 2012;3:109-16.
- Goud RN, Agarval D, Nadagoudar PH, addad SM. Antibiotic sensitivity pattern of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) in High Schools, Bangalore city, Karnataka, South India. Int Med J Stud Res 2011;1:27-35.
- Rajaduraipandi K, Mani KR, Panneerselvam K, Mani M, Bhaskar M, Manikandan P. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus: A multicentre study. Indian J Med Microbiol 2006;24:34-8.
- Tiwari HK, Das AK, Sapkota D, Sivrajan K, Pahwa VK. Methicillin resistant Staphylococcus aureus: Prevalence and antibiogram in a tertiary care hospital in western Nepal. J Infect Dev Ctries 2009;3:681-4.
- Mahmood Tahir T, Jameel T, Ziauddin A, Aslam HF. Incidence of methicillin-resistant Staphylococcus Aureus (MRSA) causing nosocomial infection in a tertiary care Hospital. Annals 2010;16:91-6.
- Saravanan M, Nanda A. Incidence of methicillin resistant Staphylococcus aureus (MRSA) from septicemia suspected children. Indian J Sci Technol 2009;2:36-9.
- Majumder D, Bordoloi JS, Phukan AC, Mahanta J. Antimicrobial susceptibility pattern among methicillin resistant Staphylococcus isolates in Assam. Indian J Med Microbiol 2001;19:138-40.
- Menezes GA, Harish BN, Sujatha S, Vinothini K, Parija SC. Emergence of vancomycin-intermediate Staphylococcus species in southern India. J Med Microbiol 2008;57:911-2.
- Shashikala, Kanungo.R, Srinivasan S, Devi S. Emerging resistance to carbapenams in hospital acquired Pseudomonas infection. A cause for concern. Indian J Pharmacol 2006;38:287-8.
- Kousalya K, Thirumurugu S, Arumainayagam DC, Manavalan R, Vasantha J, Reddy CU. Antimicrobial resistance of bacterial agents of the upper respiratory tract in South Indian population. J Adv Pharm Technol Res 2010;1:207-15.
- Parveen RM, Khan MA, Menezes GA, Harish BN, Parija SC, Hays JP. Extended-spectrum β-lactamase producing Klebsiella pneumoniae from blood cultures in Puducherry, India. Indian J Med Res 2011;134:392-5.
- Chopra S, Mahajan G, Kaur J, Sheevani, Chopra M, Mahajan S. Septicemia due to MDR isolates in ICU: A therapeutic challenge. Asian J Pharm Health Sci 2012;2:476-80.
- Saravanan R, Arun Muthukumar GL. A surveillance study of antibiotic use in Pondicherry-2012. Int J Basic Clin Pharmacol 2012;1:202-10.