An isobolographic analysis of the antinociceptive effect of xylopic acid in combination with morphine or diclofenac
- *Corresponding Author:
- Prof. Eric Woode,
Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, College of Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
E-mail: ewoode.pharm@knust.edu.gh
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: A common practice of managing pain globally is the combination of analgesics and this is aimed at facilitating patient compliance, simplifying prescription, and improving efficacy without increasing adverse effects. Fruit extracts of Xylopia aethiopica are used traditionally in the management of pain disorders and xylopic acid (XA) present in the fruit extract have been shown to possess analgesic properties in animals. There is the likelihood of concomitant use of XA and the commonly used analgesics in traditional settings. This study, therefore, evaluated the pharmacologic interaction between XA/morphine and xylopic/diclofenac combinations. Methods: The formalin test and acetic acid writhing test were used to study the antinociceptive activity of XA, morphine, and diclofenac. The isobolographic analysis was used to study the antinociceptive interactions between XA co-administered with morphine or diclofenac. Results: Results obtained revealed that XA (10–100 mg/kg), morphine (1–10 mg/kg), and diclofenac (1–10 mg/kg) produced dose-related antinociception with different potencies in the formalin and acetic acid writhing tests. Isobolographic analysis of XA/morphine and XA/diclofenac combinations revealed potentiation of their antinociceptive effects. The degree of potentiation calculated as interaction index showed synergism for both combinations in all the nociceptive tests. Conclusion: In conclusion, the present study demonstrated synergism for the co-administration of XA with morphine or diclofenac.
Keywords
Isobologram, mice, nociception, potentiation, synergism
Introduction
Combination of different analgesics is a common practice and this strategy is aimed to achieving one or more therapeutic goals such as facilitating patient compliance, simplifying prescription, improving efficacy without increasing adverse effects, or decreasing adverse effects without loss of efficacy.[1] In Ghana, a lot of combination drugs (analgesics) are in use to manage pain and these include EFPAC (aspirin 150 mg, paracetamol 250 mg, caffeine 30 mg), Kwik Action (paracetamol 500 mg, ephedrine HCl 10 mg, caffeine 30 mg), Gebedol forte (paracetamol 500 mg, diclofenac 50 mg, chlorzoxazone 250 mg), and Inflnil (diclofenac 50 mg, paracetamol 650 mg). An inappropriate combination of the constituents of a combination product may lead to antagonism or simple additive effect. Therefore, the strategic combination of the constituents is needed in order to achieve synergism. The isobolographic analysis provides a means of assessing whether biological responses induced by mixtures of agents are greater, equal, or smaller than would have been expected on the basis of the individual activities of the components.[1] The concept of dose additivity, antagonism or supra-additivity aimed at developing new pain relief strategies, that involve the combination of different analgesics that target both central and peripheral pain pathways and also produces greater analgesia at reduced and more tolerable doses of individual drugs, have become important in disease treatment and synergism when observed in isobolographic analysis may also provide information on mechanism of drug action.[2] The principle of independent joint action (the two drugs should produce overtly similar effects through different mechanism) is used as a guide for selection of drugs for isobolographic analysis and the realization of additivity indicates that the same pathway may be activated by the constituents of the combination.[3]
To date there is no single safe and effective analgesic for the treatment of pain although some of the reasons for this is attributable to inappropriate or insufficient use of existing therapies [4?6] and there is, therefore, the likelihood that there would be co?administration of xylopic acid (XA) and other drugs such as nonsteroidal anti?inflammatory drugs (NSAIDs) and opioids in the management of pain traditionally. This may lead to potentiation or antagonism of the combination. Commonly used analgesics, opioids, and NSAIDs, come with severe side effects. Opioids (e.g., morphine), though widely used and are effective in the treatment of many pain syndromes, they produce considerable adverse effects, including constipation, sedation, respiratory depression, and nausea. Furthermore, on long?term administration, they can lose their efficacy through the development of tolerance – necessitating the use of very high doses of the drug in these cases. A further important issue is that some chronic pain states, such as neuropathic pain, are not effectively treatable with opiates.[7] NSAIDs are mainstays in acute and chronic pain management, and their beneficial actions have been linked to their ability to inhibit cyclooxygenases: Constitutive COX?1 and inducible COX?2 among others.[8,9] Long?term uses of the NSAIDs come with gastric ulceration which limits their usefulness.[3] XA (15α?hydroxy?ent?kaur?16?en?19?oic acid) [Figure 1] belongs to a diterpenoid class of compounds. It has analgesic activity [10?12] and has been reported to be relatively safer in brine shrimp exhibiting a low toxicity with LC50 of 0.5 ng/ml.[13] In the light of the above, it is evident that alternative strategies based on drug combinations need to be considered in order to solve the drawbacks of opioids and NSAIDs, especially with agents with low toxicity profile such test chambers (a perspex chamber 15 cm × 15 cm × 15 cm) for 30 min before formalin injection. Male mice were then pretreated with vehicle (10 ml/kg of 0.9% NaCl, i.p), XA (10–100 mg/kg, p.o.) or morphine (1–10 mg/kg, i.p.) 60 min (p.o.) or 30 min (i.p.) before intraplantar injection of 10 μl of 5% formalin. The pain response was scored for 1 h, starting immediately after formalin injection. A nociceptive score was determined for each 5?min time block by measuring the amount of time spent biting/licking of the injected paw. The average nociceptive score for each time block was calculated by multiplying the frequency and time spent in biting/licking. Data were expressed as the mean ± standard error of mean (SEM) of scores between 0 and 10 min (first phase) and 10–60 min (second phase) after formalin injection.
Isobolographic analysis of xylopic acid/morphine and xylopic acid/diclofenac combinations
Dose?response curves for the administration of XA, morphine and diclofenac were obtained using eight animals (n = 8) at each of the dose levels. A least?square linear regression analysis of the log dose?response curve allowed the calculation of the doses that produced 50% of antinociception when each drug was administered alone. ED50 values were used in the formalin and acetic acid?induced writhing tests, as the equieffective doses, for isobolographic analysis. Then dose?response curves were also obtained and analyzed after the co?administration of XA with morphine or with diclofenac in fixed ratio (1:1) combinations based on the following fractions 1/2, 1/4, and 1/8 of their respective ED50 for both formalin and writhing tests.[1,17]
Isobologram (a cartesian plot of pairs of doses that, in combination, yield a specified level of effect) was then built by connecting the theoretical ED50 of morphine or diclofenac plotted on the abscissa and XA plotted on the ordinate to obtain the additivity line. For each drug mixture, the ED50 (experimental) and its associated 95% confidence intervals were determined by linear regressional analysis of the log dose?response curve (and compared by a t?test to a theoretical additive ED50) obtained from the formula:[1]
Zadd = f (ED50) of morphine + (1−f) ED50 of xylopic acid
Where f is the fraction of the each component in the mixture.
The variance (Var) of Zadd was calculated as:
Var Zadd = f2 (Var ED50 of morphine) + (1−f)2 Var ED50 of xylopic acid.
From these variances, SEM’s were calculated and resolved according to the ratio of the individual drugs in the combination. A supra?additive or synergistic effect is defined as the effect of a drug combination that is, higher and statistically different (ED50 significantly lower) than the theoretically calculated equieffect of a drug combination in the same proportion. If the ED50’s are not statistically different, the effect of the combination is additive, and additivity means that each constituent contributes with its own potency to the total effect. The degree of interaction was calculated using fractional analysis by dividing the experimental ED50 (Zmix) by the theoretical ED50 (Zadd). A value close to 1 was considered as additive interaction. Values lower than 1 are an indication of the magnitude of supra?additive or synergistic interactions (Zmix/Zadd <1), and values higher than 1 correspond to sub?additive or antagonistic interactions.[1,3]
Statistical analysis
In the formalin and acetic acid tests, data are presented as mean ± SEM (n = 8). The time?course curves were subjected to two?way (treatment × time) repeated measures analysis of variance (ANOVA) with Bonferroni’s post?hoc test. Total nociceptive score for each treatment was calculated in the arbitrary unit as the area under the curve (AUC). Analysis of differences in AUCs was carried out by one?way ANOVA (followed by Newman–Keuls post?hoc test) with drug treatment as a between?subjects factor. ED50 values (doses for 50% of the maximal effect) for each drug were determined by nonlinear regressional analysis (iterative computer least?squares method). The fitted midpoints (ED50s) of the curves were compared statistically using F test.[18,19] GraphPad Prism for Windows version 5 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses and ED50 determinations. P < 0.05 were considered statistically significant.
Isobolographic calculations were performed with the program PharmTools Pro, version 1.27 (The McCary Group Inc., Elkins Park, PA, USA). Results are presented as mean ± SEM or as ED50 values with 95% confidence limits. The statistical analysis of the isobolograms was performed according to the Tallarida [20] and the statistical difference between experimental and theoretical values was assessed by the Student’s t?test for independent means, and the P < 0.05 were considered significant.
Results
Formalin?induced licking test
XA and morphine inhibited both neurogenic and inflammatory pain in the formalin test. XA was more potent in the inflammatory phase of the formalin test compared to the neurogenic phase. Morphine was less potent in the neurogenic phase than the inflammatory phase. Morphine was 90.4 and 19025 times more potent than XA in the first and second phases respectively [Table 1].
| Drugs | Phase 1 ED50 (mg/kg) | Phase 2 ED50 (mg/kg) |
|---|---|---|
| Morphine | 0.15 ± 0.42 | 0.0004 ± 2.3 |
| Xylopic acid | 13.56 ± 4.5* | 7.61 ± 2.3* |
*P<0.05 compared to morphine. Values are expressed as mean ± SEM (n=8). ED50s ± SEM were obtained by the least-square nonlinear regression as described in materials and methods.
SEM: Standard error of the mean
Table 1: ED50 values of morphine and xylopic acid in both phases of the formalin test.
Isobologram of xylopic acid and morphine combination
The experimental ED50 (Zmix) obtained by nonlinear regression analysis for phase 1 [Figure 2a] was 2.58 ± 0.49 mg/kg and 2.31 ± 0.81 mg/kg for phase 2 [Figure 2b] both indicating potentiation of the antinociceptive effect of the two drugs.
The degree of potentiation calculated as the interaction index by fractional analysis indicated synergism for phase 1 [Figure 2c] and phase 2 [Figure 2d]. Isobologram consisting of morphine and XA with theoretical additive ED50(Zadd) was computed as 6.86 ± 2.24 mg/kg for phase 1 [Figure 2c] and 3.81 ± 2.5 mg/kg for phase 2 [Figure 2d and Table 2].
Figure 2: Dose-response curves for xylopic acid and morphine and fractions of their combination for (a) phase 1 and (b) phase 2 of the formalin-induced licking test respectively. Isobologram for the combination of morphine and xylopic acid in (c) phase 1 and in (d) phase 2 of formalin test in mice. Filled circles (•) are the theoretical ED50’s ± standard error of mean and open circles (o), the experimental ED50’s ± standard error of mean
| Combinations | Morphine/xylopic | Morphine/xylopic |
|---|---|---|
| acid phase 1 | acid phase 2 | |
| Theoretical ED50 (mg/kg) | 6.86±2.24 | 3.81±2.5 |
| Experimental ED50 (mg/kg) | 2.58 ± 0.49* | 2.31 ± 0.81 |
| Interaction index | 0.38 | 0.61 |
| Drugs ratio | 1:89.7 | 1:19025 |
*P<0.05 compared theoretical ED50. Values are expressed as mean ± SEM (n=8). The values were obtained from experiments described earlier. ED50s ± SEM were obtained by the least-square nonlinear regression as described in materials and methods. SEM: Standard error of the mean.
Table 2: Theoretical and experimental ED50 values of morphine and xylopic acid in both phases of the formalin test with their computed interaction indices.
Acetic acid?induced w test
XA and diclofenac inhibited visceral pain in the acetic acid?induced writhing test during the 30 min observational period. XA (10–100 mg/kg, p.o., 60 min before) reduced the writhings induced by the acetic acid in a dose?dependent manner with an ED50 of 12.94 ± 1.24 mg/kg. Diclofenac (1–10 mg/kg, i.p., 30 min before) also dose?dependently reduced the total number of writhes with an ED50 of 1.46 ± 0.27 mg/kg [Table 3].
| Drugs | ED50(mg/kg) |
|---|---|
| Xylopic acid | 12.94 ± 1.24*** |
| Diclofenac | 1.46 ± 0.27 |
***P<0.001 compared to diclofenac. Values are expressed as mean ± SEM (n=8). ED50s ± SEM were obtained by the least-square nonlinear regression as described in materials and methods.
SEM: Standard error of the mean
Table 3: Prescribing indicators at the outpatient department of ODCH (original).
Isobologram of xylopic acid and diclofenac combination
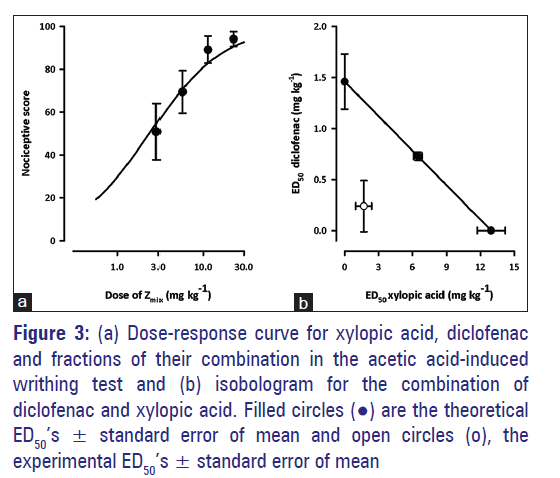
The experimental ED50 obtained after administration of XA and diclofenac and in a fixed ratio of their combination in the acetic acid?induced writhing test was 1.944 ± 0.97 [Figure 3a]. The degree of potentiation calculated as the interaction index by fractional analysis indicated synergism for the combination and graphically displayed as Zmix’s lying below the line of additivity [Figure 3b] of the isobologram. The theoretical additive ED50(Zadd) was computed as 7.201 ± 0.403 mg/kg [Table 4].
Figure 3: (a) Dose-response curve for xylopic acid, diclofenac and fractions of their combination in the acetic acid-induced writhing test and (b) isobologram for the combination of diclofenac and xylopic acid. Filled circles (•) are the theoretical ED50’s ± standard error of mean and open circles (o), the experimental ED50’s ± standard error of mean
| Zadd(mg/kg) | Zmix(mg/kg) | Interaction index | Drug ratio |
|---|---|---|---|
| 7.201 ± 0.403 | 1.944 ± 0.97** | 0.27 | 1:3.70 |
**P<0.01 compared to theoretical ED50 (Zadd). Values are expressed as mean ± SEM (n=8). The values were obtained from experiments described earlier. ED50s ± SEM were obtained by the least-square nonlinear regression as described in materials and methods.
SEM: Standard error of the mean.
Table 4: Theoretical and experimental ED50 ± SEM of xylopic acid in the acetic acid-induced writhing test and their computed interaction indices
Discussion
Administration of XA/morphine and XA/diclofenac combinations produced antinociceptive effects in the formalin and acetic acid?induced writhing tests which were greater than would be achieved for the administration of the individual drugs alone. The formalin test is one of the most predictive of acute pain and a valid model of clinical pain.[21,22] The formalin test produces two distinct phases of nociceptive response. The first phase (neurogenic phase) of paw licking/biting response is considered to be a result of the direct stimulation of C?fibre nociceptors by the injected formalin. The second phase (inflammatory phase), which appears later, is considered to be due to the combination of an inflammatory reaction in the peripheral tissue and changes in central processing.[23] Drugs that act primarily as central analgesics (e.g., opioids) inhibit both phases, whereas peripherally acting drugs (e.g., most NSAIDs and corticosteroids) inhibit only the second phase. [22,24] Inhibition of the late phase is due to inhibition of inflammatory mediators such as serotonin, histamine, bradykinin, and prostaglandins, which at least to some degree, can cause sensitization of the central nociceptive neurons.[25] XA and morphine co?administration produced a synergistic antinociceptive effect in both phases of the formalin test. Based on the principle of independent joint action, co?administration of XA and morphine activated different pathways to produce synergistic effects in both phases of the formalin test since additivity would at best be realized if only a single pathway was activated. It has previously been reported that XA inhibited pain in the formalin test by interacting with opioidergic, NO?cGMP, serotoninergic, muscarinic cholinergic, adenosinergic, and adrenergic systems while morphine inhibited pain in this model by interacting with opioidergic?ATP?sensitive K+ channels?NO?cGMP system.[11] It is worth noting, however, that the synergism between XA and morphine was lower in the inflammatory phase than a neurogenic phase. One of the possible reasons may be that the greater number of common pathways may have been shared by XA and morphine in the inhibition of inflammatory pain than neurogenic pain. Again, the combination may have activated more different pathways in the first than the second phase. The exact mechanism(s) of the combination needs to be elucidated in further studies. Nonetheless, this study has revealed that lower doses of morphine and XA can be used to achieve the greater analgesic effect while minimizing their side effects.
XA and diclofenac combination also produced synergism in the acetic acid?induced visceral pain model, and this is in agreement with the results obtained from the inflammatory phase of the formalin test. Pain produced in this model was due to peripheral and central sensitization of nociceptors by inflammatory pain mediators. Diclofenac, an example of an NSAID is known to block pain through the inhibition of prostaglandin biosynthesis, modulations of endogenous opioids, serotoninergic and noradrenergic mechanisms, and the inhibition of NO/cGMP pathway.[26,27] The inhibition of pain in the acetic acid?induced writhing test was higher than that seen in the second phase of formalin test although both share similar pathophysiology. This may be due to the aptitude of diclofenac/XA combination to inhibit production of inflammatory pain mediators as well as inhibiting the neuronal sensitizing effect of prostaglandin (if any is still produced), providing an efficient and systematic pain inhibition compared to morphine/XA combination.
XA has a low toxicity profile and fractional analysis has revealed that use of lower doses of XA with morphine or diclofenac produced synergistic antinociceptive effects. This makes the combinations useful agents compared to the use of the drugs individually for the treatment of both neurogenic and inflammatory pain conditions.
Conclusion
XA and morphine combination exhibited marked potentiation after isobolographic analysis of the combination in neurogenic and inflammatory phases of the formalin test. The degree of potentiation calculated as the interaction index revealed synergism in both phases of the formalin test. Similarly, XA and diclofenac combination also exhibited marked potentiation after isobolographic analysis of the combination in the acetic acid?induced writhing test. The degree of potentiation calculated as the interaction index revealed synergism of the combination in acetic acid?induced writhing test.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Tallarida JR. Drug Synergism and Dose-Effect Analysis. 1st ed. Boca Raton: Chapman and Hall/CRC; 2000.
- Tallarida RJ, Stone DJ Jr, McCary JD, Raffa RB. Response surface analysis of synergism between morphine and clonidine. J Pharmacol Exp Ther 1999;289:8-13.
- Miranda HF, Puig MM, Dursteler C, Prieto JC, Pinardi G. Dexketoprofen-induced antinociception in animal models of acute pain: Synergy with morphine and paracetamol. Neuropharmacology 2007;52:291-6.
- McMahon SB, Koltzenburg M. Wall and Melzack’s Textbook of Pain. 5th ed. Edinburgh: Elsevier/Churchill Livingstone; 2006.
- Chen CH, Tang ST, Chen CH. Meta-analysis of cultural differences in Western and Asian patient-perceived barriers to managing cancer pain. Palliat Med 2012;26:206-21.
- Acute pain, Bandolier Extra-Evidence based health care 2003. p. 1-22.
- Pelissier T, Laurido C, Kramer V, Hernández A, Paeile C.Antinociceptive interactions of ketamine with morphine or methadone in mononeuropathic rats. Eur J Pharmacol 2003;477:23-8.
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011;31:986-1000.
- Warner TD, Mitchell JA. Cyclooxygenases: New forms, new inhibitors, and lessons from the clinic. FASEB J 2004;18:790-804.
- Ameyaw EO, Woode E, Boakye-Gyasi E, Abotsi WK, Kyekyeku JO, Adosraku RK. Anti-allodynic and anti-hyperalgesic effects of an ethanolic extract and xylopic acid from the fruits of Xylopia aethiopica in murine models of neuropathic pain. Pharmacognosy Res 2014;6:172-9.
- Woode E, Ameyaw EO, Ainooson GK, Abotsi WK, Boakye-Gyasi E, Kyekyeku JO. Analgesic effects of an ethanol extract of the fruits of Xylopia aethiopica and xylopic acid in murine models of pain: Possible mechanism(s). Pharmacologia 2013;4:285-300.
- Woode E, Ameyaw EO, Boakye-Gyasi E, Abotsi WK. Analgesic effects of an ethanol extract of the fruits of Xylopia aethiopica (Dunal) A. Rich (Annonaceae) and the major constituent, xylopic acid in murine models. J Pharm Bioallied Sci 2012;4:291-301.
- Somova LI, Shode FO, Moodley K, Govender Y. Cardiovascular and diuretic activity of kaurene derivatives of Xylopia aethiopica and Alepidea amatymbica. J Ethnopharmacol 2001;77:165-74.
- Adosraku RK, Kyekyeku JO. Characterisation and HPLC quantification of xylopic acid in the dried fruits of Xylopia Aethiopica. Int J Pure Appl Chem 2011;6:209-13.
- Woode E, Abotsi WK. Antinociceptive effect of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae). J Pharm Bioallied Sci 2011;3:384-96.
- Kabir A, Samad MB, D'Costa NM, Hannan JM. Investigation of the central and peripheral analgesic and anti-inflammatory activity of Draksharishta an Indian Ayurvedic formulation. J Basic Clin Pharm 2012;3:336-40.
- Miranda HF, Sierralta F, Prieto JC. Synergism between NSAIDs in the orofacial formalin test in mice. Pharmacol Biochem Behav 2009;92:314-8.
- Miller JR. GraphPad Version 4.0. Step-by-Step Examples. San Diego: GraphPad Software Inc.; 2003.
- Motulsky HJ, Christopoulos A. Fitting Model to Biological Data Using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. San Diego: GraphPad Software Inc.; 2003.
- Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther 2006;319:1-7.
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev 2001;53:597-652.
- Hunskaar S, Hole K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987;30:103-14.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: An evaluation of the method. Pain 1992;51:5-17.
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, et al. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol 2001;90:2386-402.
- Santa-Cecilia FV, Vilela FC, da Rocha CQ, Dias DF, Cavalcante GP, Freitas LA, et al. Anti-inflammatory and antinociceptive effects of Garcinia brasiliensis. J Ethnopharmacol 2011;133:467-73.
- Diaz-Reval MI, Ventura-Martinez R, Deciga-Campos M, Terrón JA, Cabré F, Lopez-Munoz FJ. Evidence for a central mechanism of action of S-(+)-ketoprofen. Eur J Pharmacol 2004;483:241-8.
- Fürst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull 1999;48:129-41.