An antioxidant potential of hydromethanolic extract of Urtica parviflora Roxb.
- *Corresponding Author:
- Sangeeta Pilkhwal Sah
Department of Pharmaceutical Sciences, Bhimtal Campus, Kumaon University, Nainital, Uttarakhand
E-mail: spilkhwal@yahoo.com
Date of Received : 26-03-2010
Date of Modefied : 20-04-2010
Date of Accepted : 03-05-2010
Available Online : 15-08-2010
Abstract
Antioxidant activity of hydromethanolic extract of Urtica parviflora Roxb. (family Urticaceae) was investigated by different in vitro methods, namely, nitric oxide scavenging, DPPH scavenging, and reducing power assay. In the present study, plant extract exhibited dose dependent free radical scavenging and reducing activity. The antioxidant activity of the hydromethanolic extract of Urtica parviflora Roxb. was compared with ascorbic acid as standard. In addition, phytochemical screening of hydromethanolic extract of the plant was undertaken to identify the phytochemicals present in the extract. Phytochemical examination revealed the presence of alkaloids, polysaccharides, saponins, flavonoids, phenolic compounds, glycosides and tannins. It was concluded that the extract contains important phytoconstituents responsible for antioxidant effect. The study indicated that Urtica parviflora could protect the cell injury caused by the reactive oxygen species and might be a valuable source of antioxidant both for medicine and food industry.
Keywords
Urtica parviflora, flavonoids, alkaloids, DPPH scavenging, reducing power
Introduction
Free radicals have aroused signifi cant interest among scientists in the past decades because of their broad range of eff ects on biological systems. Reactive oxygen species (ROS) like superoxide anion radical, hydroxyl radical, hydrogen peroxide and nitric oxide are continuously formed inside the body. However, normally a balance between oxidative events and antioxidative forces maintains the status quo within living cells. When normal balance is upset, either by loss of reducing agents or protective enzymes or by boThevents simultaneously, the tissue is considered to be under oxidative stress. It can then cause oxidative damage of all major groups of biomolecules (DNA, proteins, lipids and small cellular molecules) leading to pathogenesis of various diseases like cancer, emphysema, cirrhosis, atherosclerosis, arthritis cardiovascular diseases, diabetes, asthma, hepatitis, liver injury, immune defi ciency diseases, neurodegenerative diseases and aging [1-4].
Due to the above-presented pathological implications of ROS, it is important to fi nd an antioxidant, which may scavenge multiple ROS so that it can be used in multiple disease states and also to maintain a healthy states. Butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are extensively used in food industries as antioxidants. However, the possible toxicity as well as general consumer rejection led to decreasing use of these synthetic antioxidants [5].
Flavonoids and other phenolic compounds of plant origin have been reported as scavengers of free radicals [6]. Nowadays search for natural antioxidant source is gaining much importance. Therefore, attempt has been made to evaluate antioxidant potential of Urtica parviflora. Roxb. in the present study. Urtica parviflora Roxb. belongs to the family Urticaceae and is widely distributed throughout the India, especially in Himalaya (lower altitude) from Kashmir to Sikkim, in Darjeeling, West Bengal, Mishmi hills in Arunachal Arunachal Pradesh and Nilgiri hills in the south [7]. It is commonly known as Himalayan stinging nettle and locally as ‘Shishoon’ in Kumaun and ‘Kaldiya’ or ‘Kandali’ in Garhwal. Its leaves and stems produce infl ammatory rash, accompanied by a considerable burning and itching sensation attributed to the presence of histamine and 5-hydroxytryptamine [8]. The roots are employed for the treatment of fractures of bone and dislocations of joints [7]. The leaves are used in dysentery, joint pain and liver disorders [9]. The inflosescense are used as cleansing agent after parturition and in the treatment of dermatitis in alpine region of central and eastern Himalayas [7].
Experimental
Plant material
The plant material was collected from local surroundings of Bhimtal (Nainital), India in the month of September 2009 and was identified from Botanical Survey of India, Dehradun. A voucher specimen (No.112286) has been kept in Department of Pharmaceutical Sciences, Bhimtal Campus, Kumaun University, Nainital.
Preparation of extract
The air dried leaves of Urtica parviflora (20 g) were extracted with 100 ml of solvent (methanol : water, 4:1). The resultant extract was concentrated under reduced pressure to yield a green residue.
Drugs and Chemicals
Potassium ferricyanide, sodium nitroprusside, ferric chloride and trichloroacetic acid. Sulfanilamide, napthylethylene diamine hydrochloride, orthophosphoric acid and 1,1-diphenyl 2-picryl hydrazyl (DPPH) were obtained from Sigma Chemicals, USA. Ascorbic acid was procured from Ranbaxy, India. All other unlabelled chemicals and reagents were of analytical grade and were used without further purification.
Phytochemical Screening
The hydromethanolic extract was qualitatively tested for the presence of chemical constituents. Phytochemical screening was performed using following reagents and chemicals. Alkaloids with Dragendorff 's reagent; phenolic compounds with FeCl3; glycosides with glacial acetic acid, FeCl3 and Conc. H2SO4; fl avonoids with magnesium chip and HCl; tannins with lead acetate and 5% ferric chloride; polysaccharides with iodine test; triterpenoids with Liebermann-Burchardt’s test and saponins with ability to produce suds. These were identified by characteristic color changes using standard procedures [10].
In vitro screening for antioxidant activity
DPPH radical scavenging assay
DPPH (1,1-diphenyl-2-picryl hydrazyl) scavenging activity was measured by spectrophotometric method. 2.95 ml of methanolic solution of DPPH (100 μM) was added to 0.05 ml of diff erent concentrations (10-640 μg/ml) of hydromethanolic extract of Urtica parviflora dissolved in dimethylsulfoxide (DMSO). Equal amount of DMSO was added to the control. Absorbance was recorded at 517 nm after 20 min [11]. Ascorbic acid was used as a standard and all the assays were carried out in duplicate. The purple colour of DPPH changes to yellow, based on the effi - cacy of antioxidants. Radical scavenging activity was expressed as percent inhibition and was calculated using the following formula.
Percentage inhibition (%) = [(Acontrol – Asample) / Acontrol)]x100.
where, Acontrol is the absorbance of control reaction (containing all reagents except test compound), and Asample is the absorbance of test compound. IC50 values (concentration of sample required to scavenge 50% of free radicals) were calculated from the regression equation, prepared from the concentration of the samples and percentage inhibition of free radical formation.
Nitric Oxide scavenging assay
Sodium nitroprusside (5 mM) in phosphate buff er saline was mixed with diff erent concentrations of hydromethanolic extract (10-640 μg /ml) dissolved in DMSO and incubated at 25oC for 30 min. After 30 min, 1.5 ml of incubated solution was removed and diluted with 1.5 ml of Griess reagent (1% sulphanilamide, 2% orthophosphoric acid and 0.1% napthylethylene diamine dihydrochloride). The absorbance of the chromophore formed during diazotisation of the nitrite with sulphanilamide and subsequent coupling with napthylethylene diamine was measured at 546 nm along with a control [12]. The percentage inhibition of nitric oxide generated was measured by comparing the absorbance values of control and test samples using following formula.
Percentage inhibition (%) = [(Acontrol – Asample) / Acontrol)]x100.
where, Acontrol is the absorbance of the control reaction (containing all reagents except test compound), and Asample is the absorbance of test compound. IC50 values (concentration of sample required to scavenge 50% of free radicals) were calculated from the regression equation, prepared from the concentration of the samples and percentage inhibition of free radical formation. Ascorbic acid was used as positive control and all tests were carried out in duplicate.
Reducing power
Reductive ability of the extract was measured according to the method of Oyaizu [13]. Diff erent concentrations (10-640 μg /ml) of extract were mixed with 2.5 ml of sodium phosphate buff er (pH 6.6) and 2.5 ml of potassium ferricyanide (1%). The mixture was incubated at 50°C for 20 min. Trichloroacetic acid (2.5 ml of 10%) was added to it, the mixture was mixed and centrifuged at 650 rpm for 10 min. The upper layer (5 ml) was mixed with 5 ml of deionised water and 1 ml of ferric chloride (1%) and absorbance was measured at 700 nm. Control reaction contains all the reagents except test compound. Higher absorbance indicated higher reducing power.
Results
Phytochemical screening
The yield of residue obatined after extraction was found to be 8.9% w/w. Phytochemical screening revealed the presence of alkaloids, polysaccharides, saponins, flavonoids, tannins, phenolic compounds and glycosides in the Urtica leaves (Table 1).
| Chemical Constituents | Test | |
|---|---|---|
| Alkaloids | Dragendroff’s reagent | + + |
| + + | ||
| Saponins | Foam test | |
| + + | ||
| Flavonoids | Magnesium and HCl | |
| + + | ||
| Phenolic compounds | FeCl3 test | |
| + + | ||
| Glycosides | Test with glacial acetic acid, FeCl3, H2SO4 dropwise | |
| Tannins | FeCl3 Lead acetate | + + |
| + + | ||
| Steroids/triterpenoids | Liebermann-Burchardt’s test | |
| - | ||
++ = copiously present; - = absent.
Table 1: Phytochemical screening of hydromethanolic extract of leaves of U. parviflora
DPPH radical scavenging assay
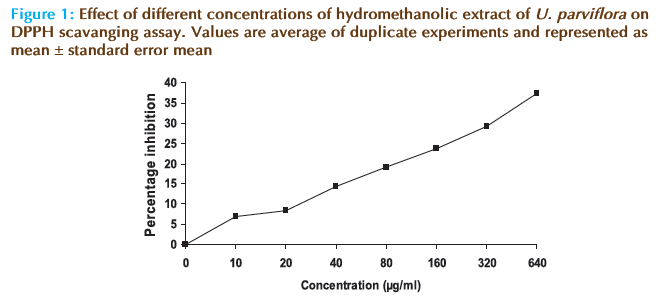
Free radical scavenging potential of extract at different concentrations was tested by DPPH method and the results are depicted in Figure 1. The results showed that the hydromethanolic extract of U. parviflora has reduced the free radical (1,1-diphenyl- 2-picrylhydrazyl) to corresponding hydrazine in a concentration dependent manner. IC50 values were found to be 808 μg/ml for hydromethanolic extract and 22.43 μg/ml for ascorbic acid. The results thus demonstrated good free radical scavenging activity of the extract.
Nitric oxide scavenging effect
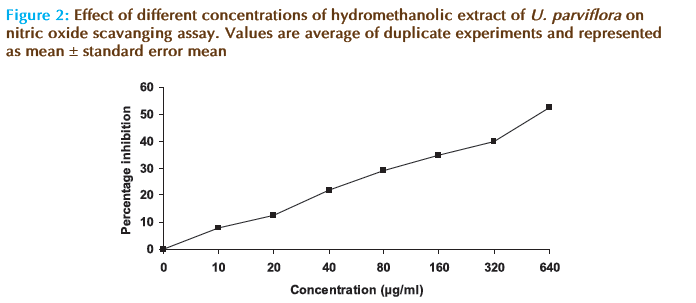
The hydromethanolic extract of U. parviflora eff ectively reduced the generation of nitric oxide radicals from sodium nitroprusside solution in a concentartion dependent manner (Figure 2). Th is showed signifi cant nitric oxide scavenging activity of the extract (IC50 =519.15 μg/ml). IC50 of ascorbic acid was found to be 30 ± 1.82 μg/ml.
Reducing Power
The extract exhibited concentration dependent increase in absorbance. Absorbance indicated by all the concentrations of extract was signifi cantly higher than the absorbance of control reaction (0.031). Higher absorbance indicates high reducing power due to formation of reduced intermediate.
Table 2 shows the reductive capability of the plant extract compared to ascorbic acid. Ascorbic acid has much higher reducing ability than the hydromethanolic extract.
| Concentration (μg/ml) | Absorbance at 700 nm | ||
|---|---|---|---|
| Ascorbic acid | U. parviflora extract | ||
| 10 | 0.059 | 0.042 | |
| 20 | 0.099 | 0.052 | |
| 40 | 0.252 | 0.059 | |
| 80 | 0.457 | 0.062 | |
| 160 | 0.875 | 0.078 | |
| 320 | 1.668 | 0.118 | |
| 640 | 2.648 | 0.215 | |
Table 2: Reducing ability of different concentrations of hydromethanolic extract of U. parviflora. Values are average of duplicate experiments. Ascorbic acid was taken as standard.
Discussion
In the present study we investigated the antioxidant activity of the hydromethanolic extract of Urtica parviflora in some in vitro antioxidant models. In all the models, extract showed its ability to scavenge the free radicals in a concentration dependent manner.
The free radical scavenging activity was studied by its ability to reduce the stable radical DPPH. Antioxidants react with DPPH, a nitrogen-centered radical due to their hydrogen donating ability and convert it to 1,1,-diphenyl-2-picryl hydrazine [14].
The degree of discoloration indicates the scavenging potential of the antioxidant. From the DPPH assay it may be postulated that the extract reduces the radical to the corresponding hydrazine and this scavenging ability of the extract may be attributed to its hydrogen donating ability.
Nitric oxide is a potent pleiotropic mediator of physiological processess as well as it is involved in pathogenesis of pain and infl ammation. Our study demonstrated a potent nitric oxide scavenging activity of U. parviflora extract and off ers a scientifi c evidence that the plant can be used in infl ammatory conditions.
The reducing ability of a compound generally depends on the presence of reductants [15], which exhibit antioxidative potential by breaking the free radical chain, donating a hydrogen atom [16]. The presence of reductants (i.e. antioxidants) in U. parviflora extract might have caused the reduction of Fe3+/ ferricyanide complex to the ferrous form (Fe2+) which was monitored by measuring the formation of Perl’s Prussian blue at 700 nm. However the reducing power of extract is much less compared to ascorbic acid.
Results of phytochemical screening revealed the presence of chemical constituents like alkaloids, polysaccharides, saponins, flavonoids, glycosides and tannins in large amount in hydromethanolic extract. Antioxidant activity of U. parviflora thus may be contributed to the presence of fl avonoids, phenolic compounds, alkaloids and glycosides as they possess signifi cant antioxidant activity [17-20]. Th is in vitro antioxidant activity of the extract is further supported by other workers who reported that the extract of U. parviflora signifi cantly protected the experimental animal against CCl4-induced hepatotoxicity [21].
Conclusion
Overall, it could be concluded that U. parviflora leaves bear a potent antioxidant activity as their constituents scavenge free radicals and have reducing activities. The phenolic compounds, flavonoids and alkaloids present in the extract may be responsible for antioxidant activity. Thus it can be inferred that U. parviflora extract, owing to its free radical scavenging ability can be used as a source of natural antioxidants with potential application to reduce oxidative stress with health benefits. Further investigations are necessary for encompassing in-vivo antioxidant activity of the studied plant.
Acknowledgements
The authors like to express their thanks to Dr. Laxam Singh Rautela, Lab Technician, Department of Pharmaceutical Sciences, Kumaun University Nainital, India for his help in conducting the spectrophotometric analysis.
References
- Lee K, Mitchell AE and Shibamoto T. Determination of antioxidant properties of aroma extracts from various beans. J. Agric. Food Chem. 2000; 48(10): 4817-20.
- Middleton E, Kandaswamy C and Theoharides T. The effects of plant fl avonoids on mammalian cells: Implications for infl ammation, heart disease, and cancer. Pharmacol. Rev. 2000; 5(4): 673-751.
- Pietta P, Simonetti P and Mauri P. Antioxidant activity of selected medicinal plants. J. Agric. Food Chem. 1998; 46 (11): 4487-90.
- Halliwell B. Establishing the signifi cance and optimal intake of dietary antioxidants:the biomarker concept. Nutr. Rev. 1999; 57(4):104-13.
- Branien AL. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc. 1975; 52 (2): 59-63.
- Rice-Evans C, Miller N and Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997; 2(4):152-9.
- Ramachandran K, Wealth of India (Raw Materials). New Delhi, Publications and Information Directorate, Council of Scientifi c and Industrial Research; 1992.
- Saxena PR , Pant MC, Kishar K, et al. Pharmacologically active constituents of Urtica parvifl ora Roxb. Can. J. Physiol. Pharmacol. 1965; 43 (6): 869-76.
- Gurung G. The Medicinal Plants of Sikkim Himalaya. Sikkim, Subhash Publication; 1999.
- Kokate CK. Practical Pharmacognosy. Nirali Prakashan;1994.
- Sreejayan N and Rao MNA. Free radical scavenging by curcuminoids. Arzneimittelforschung. 46(2):169-71.
- Sreejayan N and Rao MNA. Nitric oxide scavenging by curcuminoids. J.f Pharm. Pharmacol. 1997; 49(1):105-7.
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986; 44:307-15.
- Yamaguchi T, Takamura H, Matoba T, et al. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci. Biotechnol Biochem. 1998; 62(6):1201-4.
- Duh PD, Tu YY and Yen GC. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum moifolium Ramat). Lebensm Wiss Technol.1999; 32(5): 269-77.
- Gordon MH. The mechanism of the antioxidant action in vitro. In:Hudson BJF, eds. Food Antioxidants. London, Elsevier, 1990: 1-18.
- Vaya J, Belinky PA and Aviram M. Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Rad. Biol. Med. 1997; 23(2): 302-13.
- Lucia R, Magdaléna M, Daniela K, et al. Antiradical and antioxidant activities of alkaloid isolated from Mahonia aquifolium. Structural aspects. Bioorg. Med. Chem. 2004;12(17): 4709-15.
- Jagan Mohan Rao L, Ramalakshmi K, Borse BB, et al. Antioxidant and radical-scavenging carbazole alkaloids from the oleoresin of curry leaf (Murraya koenigii Spreng.). Food Chem. 2007;100(2): 742-47.
- Janbaza KH, Saeed SA and Gilanib AH. Protective effect of rutin on paracetamol and CCl4-induced hepatotoxicity in rodents. Fitoterapia. 2002; 73(7-8): 557-63.
- Prasana KK, Lilakanth N, Suvakanta D, et al. Hepatoprotective effect of the ethanolic extract of Urtica parvifl ora Roxb. in CCl4 treated rats. Int. J. Pharmacol. 2007; 3: 362-66.