A review on proniosomal drug delivery system for targeted drug action
- *Corresponding Author:
- Dr. G. V. Radha
Department of Pharmaceutics, Gandhi Institute of Technology and Management, Institute of Pharmacy, Gandhi Institute of Technology and Management, University, Rushikonda, Visakhapatnam - 530 045, Andhra Pradesh, India.
E-mail: radhagadela@gmail.com
Abstract
Proniosomes are dry formulation of water soluble carrier particles that are coated with surfactant. They are rehydrated to form niosomal dispersion immediately before use on agitation in hot aqueous media within minutes. Proniosomes are physically stable during the storage and transport. Drug encapsulated in the vesicular structure of proniosomes prolong the existence of drug in the systematic circulation and enhances the penetration into target tissue and reduce toxicity. From a technical point of view, niosomes are promising drug carriers as they possess greater chemical stability and lack of many disadvantages associated with liposomes, such as high- cost and variable purity problems of phospholipids. The present review emphasizes on overall methods of preparation characterization and applicability of proniosomes in targeted drug action.
Keywords
Characterization, clinical applications, niosomes, proniosomes, targeted drug action
Introduction
In recent times, no single drug delivery system fulfills all the criteria, but attempts have been made through novel approaches. Many novel approaches emerged covering various routes of administration, to achieve either controlled or targeted delivery. The prime aim of novel drug delivery is maintenance of the constant and effective drug level in the body and minimizing the side-effects and it also localizes the drug action by targeting the drug delivery by using drug carriers.
Vesicular drug delivery is one of the approaches, which encapsulate the drug e.g.: Liposomes, niosomes, transferosomes, pharmacosomes, and provesicles such as proniosomes and proliposomes. Advantages of liposomes and niosomes over other conventional dosage forms are their particulate nature, which act as a drug reservoir. Few modifications can also be carried out in order to adjust the pattern and the drug release. It was also found out that modified vesicles had properties that successfully delivered drugs into deeper layers of the skin. [1]
From early 1980s, proniosomes have gained wide attention by researchers for their use as drug targeting agents and drug carriers to have a variety of merits while avoiding demerits associated with the conventional form of drugs. Niosomes are water soluble carrier particles, and these are dried to form a niosomal dispersion on brief agitation in hot aqueous media. This dehydrated product is called proniosomes. The resulting niosomes are very correlative to conventional niosomes and of higher size uniformity. The proniosomal approach reduces the problems associated with dry, freeflowing product, which is more stable during the storage and sterilization. The proniosomes are a versatile delivery system because of the ease of distribution, measuring, transfer, and storage. [2]
Proniosomes were studied as alternatives to liposomes and other carrier systems for entrapping both polar and nonpolar or hydrophobic and hydrophilic drugs. The additional merits with proniosomes are low toxicity owing to non-ionicnature, no requirement of special precautions and conditions for formulation and preparations. In addition, it is the simple method for the routine and large scale production of proniosomes without the use of undesirable solvents. However, stability is a main concern in the advancement of any formulation and even proniosomes have advantages as drug carriers, such as cost productivity, chemically stability in comparision to liposomes. They also minimize problems of physical stability such as fusion, leakage, sedimentation, and aggregation on storage.
All these advantages of dry niosomes often termed as proniosomes have made them a promising industrial product.
Structure
Proniosomes are microscopic lamellar structures. They combine a non-ionic surfactant of the alkyl or dialkyl polyglycerol ether class and cholesterol followed by hydration in aqueous media. The surfactant molecule direct themselves such that the hydrophilic ends of the non-ionic surfactant orient outward, while the hydrophobic ends are in the opposite direction to form the bilayer. Like liposomes proniosomes are also made up of a bilayer. In proniosomes the bilayer is made of non-ionic surface active agents. [2]
On the basis of method of preparation proniosomes are unilamellar or multi-lmellar. The niosome is made of a surfactant bilayer with its hydrophilic ends exposed on the outside and inside of the vesicles while the hydrophobic chains face each other within the bilayer. Hence the vesicle holds hydrophilic drugs within the space enclosed in the vesicle and the hydrophobic drugs are embedded within the bilayer.
Types of Vesicular Drug Delivery Systems
1. Liposomes
2. Virosomes
3. Niosomes
4. Proniosomes
5. Transferosomes
6. Proteasomes
7. Sphingosomes
8. Archaesome
9. Ethosomes
Liposomes
Liposomes are artificially prepared vesicle composed of a bilayer of lipids. These can be used as a vehicle for administration of nutrients and pharmaceutical drugs. Liposomes can be prepared by disrupting biological membranes. They are composed of natural phospholipids and may also contain mixed lipid chains with surfactant properties. [3]
Virosomes
Virosomes are drug or vaccine delivery mechanism consisting of unilamellar phospholipid membrane, which is either a mono or bi-layer vesicle incorporating virus derived proteins to allow the virosomes to fuse with target cells. [4]
Niosomes
A niosome is a non-ionic surfactant-based liposome. Niosomes are formed mostly by cholesterol incorporation as an excipient. Other excipients can also be used. Niosomes have more penetrating capability than the previous preparations of emulsions. They are structurally similar to liposomes in having a bilayer; however, the materials used to prepare niosomes make them more stable and thus niosomes offer many more advantages over liposomes. [5]
Proniosomes
Proniosomes are dry formulation of water soluble carrier particles that are coated with surfactant. They are rehydrated to form niosomal dispersion immediately before use on agitation in hot aqueous media within minutes. Proniosomes are physically stable during storage and transport. Drug encapsulated in vesicular structure of proniosomes prolong the existence of drug in the systematic circulation and enhances the penetration into target tissue and reduce toxicity. [6]
Transferosomes
A transferosome carrier is an artificial vesicle designed to be such as a cell vesicle or a cell engaged in exocytosis and thus suitable for controlled and potentially targeted drug delivery. [7]
Proteasomes
Proteasomes are cytoplasmic organelle, composed of a cylindric core particle bound by two regulatory particles at each end, responsible for degrading endogenous proteins. Proteins to be destroyed are recognized by proteasomes because of the presence of ubiquitin conjugated to the targeted protein’s lysine residue. [8]
Sphingosomes
Sphingosomes is bilayered vesicles in which an aqueous volume is entirely enclosed by membrane lipid bilayer mainly composed of natural or synthetic sphingolipid. Sphingosomes solve the major drawback of vesicle system (liposomes, niosomes) such as less stability, less in vivo circulation time, low tumor loading efficacy in case of cancer therapy. Sphingosomes are clinically used delivery system for chemotherapeutic agent, biological macromolecule and diagnostics. Owing to flexibility in size and composition, different types of sphingosomes have been developed. [9]
Archaesome
Archaeosomes are liposomes made from the polar ether lipids of Archaea. These lipids are unique and distinct in structure from the ester lipids found in Eukarya and Bacteria. [10]
Ethosomes
Ethosomes are soft, malleable vesicles tailored for enhanced delivery of active agents. It has been shown that the physicochemical characteristics of ethosomes allow this vesicular carrier to transport active substances more efficaciously through the stratum corneum into the deeper layers of the skin than conventional liposomes. [11]
Action of Proniosomes
Proniosomes show their action after they are converted to niosomes on hydration. Proniosomes → Niosomes (on hydration). The hydration may occur by the addition of aqueous solvents. Proniosomes can entrap both hydrophilic and lipophilic drugs [Table 1]. [12]
| Materials | Specification | Action |
|---|---|---|
| Span and tween | Surfactants | Maintains HLB level |
| Cholesterol and | Membrane | Cholesterol: Influences the |
| lecithin | stabilizers | stability and permeability of |
| vesicles | ||
| Lecithin: Penetration enhancer | ||
| Maltodextrin, lactose, | Carriers | Holds the drug |
| sorbitol, mannitol | ||
| Methanol, chloroform, | Organic | Influence on vesicle size and |
| ethyl alcohol | solvents | permeability of drug |
HLB: Hydrophilic lipophilic balance
Table 1: Different materials used and their action in the preparation of proniosomes
Types of Proniosomes
According to the type of carrier and method of preparation of proniosomes they are of two types.
Dry granular proniosomes
1. Sorbitol based proniosomes
2. Maltodextrin based proniosomes
Sorbitol based proniosomes is a dry formulation that involves sorbitol as a carrier, which is further coated with non-ionic surfactant and is used as a noisome within minutes by the addition of hot water followed by agitation.. [1]
Maltodextrin based proniosomes are prepared by fast slurry method. [1]
Liquid crystalline proniosomes
This type of proniosomes are reservoirs for transdermal delivery of the drug. The transdermal patch involves an aluminum foil as a baking material along with a plastic sheet. Proniosomal gel is spread evenly on the circular plastic sheet followed by covering with a nylon mesh. [1]
Preparation of Proniosomes
The proniosomes consist of a number of ingredients such as the non-ionicsurfactant, cholesterol orlecithin being the main ingredient. Some of the methods, which were reported for the preparation of proniosomes are as follows:
1. Hand shaking method
2. Slurry method
3. Slow spray coating method
Hand shaking method
The mixture of vesicles forming ingredients such as cholesterol and surfactants are dissolved in ether, methanol or chloroform in a round bottom flask. The organic solvent evaporates at room temperature (20°C) in the rotary evaporator leaving a thin layer of solid mixture deposited on the walls of the round bottomed flask. The dried surfactant film can be rehydrated with the aqueous phase at 0-60°C with little agitation. This process produces typical multi-lamellarniosomes. [2]
Slurry method
A 250 μmol stock solution of surfactant and membrane stabilizer was prepared in chloroform:methanol (2:1) solution. A definite volume of stock solution and drug dissolved in chloroform:methanol (2:1) solution was added to a 100 ml round bottom flask containing the carrier marterial. Additional organic solvent solution added to form a slurry if lower surfactant loading occurs. The flask was attached to a rotary flash evaporator, which evaporates solvent at 60 -70 rpm, a temperature was 45 ± 2°C, and a reduced pressure of 600 mmHg until the mass in the flask had become a dry, free flowing product. These materials were dried in a desiccator overnight at room temperature under vacuum. This dry preparation is referred to as “proniosomes” and was used for preparations and for further study on powder properties. These final products ,that is proniosomes were stored in a tightly closed container at refrigerator temperature until further evaluation. [2]
Slow spray coating method
A 100 ml round bottom flask containing desired amount of carrier can be attached to rotary evaporator. The evaporator has to be evacuated and rotating flask can be rotated in a water bath under vacuum at 65-70oC for 15-20 min. This process is repeated until all of the surfactant solution has been applied. The evaporation should be continued until the powder becomes completely dry. [12,13]
Characterization of Proniosomes
Evaluation studies are further carried out for the prepared proniosomes in order to find out the
• Measurement of angle of repose
• Scanning electron microscopy (SEM)
• Optical microscopy
• Measurement of vesicle size
• Drug content
• Entrapment efficiency
• In-vivo release studies
• Stability studies
Measurement of angle of repose
The angle of repose of dried proniosomes was measured by funnel method and cylinder method.
Funnel method
The funnel, which was fixed at a position and the proniosomal powder was poured into it so that the outlet orifice of the funnel is 10 cm above the level of surface. The powder flowed down from the funnel to form a cone on the surface and then angle of repose was further calculated by measuring the height of the cone and the diameter of its base.
Cylinder method
The proniosomes powder was poured into a cylinder, which was fixed at a position so that the outlet orifice of the cylinder is 10 cm above the level of surface. The powder flowed down in the cylinder to form a cone on the surface. The angle of repose was further calculated by measuring the height of the cone and the diameter of its base. [12]
Angle of repose is calculated by the below equation
θ = Tan-1 × (h/r)
SEM
Particle size of proniosomes is a factor of prime importance. The surface morphology and size distribution of proniosomes were studied by SEM. A double-sided tape that was affixed on aluminum stubs and the proniosomal powder was spread on it. The aluminum stub was placed in a vacuum chamber of scanning electron microscope (XL 30 ESEM with EDAX, Philips, Netherlands). The morphological characterization of the samples was observed using a gaseous secondary electron detector (working pressure of 0.8 torr, acceleration voltage-30.00 KV) XL 30, (Philips, Netherlands). [15]
Optical microscopy
The niosomes were mounted on glass slides and viewed under a microscope (Medilux-207RII, Kyowa-G etner, Ambala, India). The microscope has a magnification of ×1200 used for morphological observation after sufficient dilution. The photomicrograph of the preparation was obtained from the microscope by using a digital Single lens reflex (SLR) camera. [15]
Measurement of vesicle size
The vesicle dispersions were diluted about 100 times in the same medium, which was used for their preparation. Vesicle size was measured on a particle size analyzer. The apparatus consist of a He-Ne laser beam of 632.8 nm focused with a minimum power of 5Mw using a Fourier lens (R-5) to a point at the center of multi-element detector and a small volume sample holding cell. The samples were stirred with a stirrer before determining the vesicle size. [16]
Drug content
Proniosomes equivalent to 100 mg were taken in a standard volumetric flask. They were lysed with 50 ml methanol by shaking for 15 min. The solution was diluted to 100 ml with methanol. Then 10 mlof this solution was diluted to 100 ml with saline phosphate buffer at certain pH. Aliquots were withdrawn and absorbance was measured at a certain wavelength and drug content was further calculated from the calibration curve. [17]
Entrapment effificiency
Separation of unentrapped drug from the niosomal suspension was carried out by exhaustive dialysis method and centrifugation method. Theniosomal suspension was taken into a dialysis tube to which osmotic cellulose membrane was securely attached to one side, the dialysis tube was suspended in 100 ml saline buffer at certain pH, which was stirred on a magnetic stirrer. The niosomal suspension and the unentrapped drug were seperated into the medium through osmotic cellulose membrane. After 6 h of exhaustive dialysis, optical density values were noted and the estimation of the entrapped drug was carried out by UV spectrophotometric method. Entrapment Efficiency was calculated using the formula. [18]
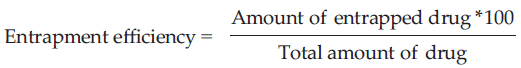
In vivo release studies
The release of the drug from the proniosomal formulations was determined using different techniques such as Franz diffusion cell, Keshary-Chien diffusion cell, Cellophane dialyzing membrane, United states pharmacopeia (USP) dissolution apparatus Type-1, spectrapor molecular porous membrane tubing. Drug release from proniosomes derived niosomal vesicles can follow any one or more of the following mechanisms; desorption from the surface of vesicles or diffusion of drug from bilayered membrane or a combined desorption and diffusion mechanisms. [19]
Stability studies
Stability studies were carried out by storing the prepared proniosomes at various temperature conditions such as refrigeration temperature (2°-8°C), room temperature (25° ± 0.5°C) and elevated temperature (45° ± 0.5°C) from a period of 1 month to 3 months. Drug content and variation in the average vesicle diameter were periodically monitored.
International conference on harmonization (ICH) guidelines suggests stability studies for the dry proniosome powders meant for reconstitution should be studied for accelerated stability at 40°C/75% relative humidity as per international climatic zones and climatic conditions (WHO, 1996). For long term stability studies the temperature is 25°C/60% RH for the countries in zone I and II and for the countries in zone III and IV the temperature is 30°C/65% Relative humidity (RH). Product should be evaluated for appearance, color, assay, pH preservative content, particulate matter, sterility, and pyrogenicity. [19]
Advantages of Proniosomes
1. Both the non-ionic surfactants and phospholipids in proniosomes can act as penetration enhancers and help in diffusion of the drug.
2. Proniosomes have higher advantages such as additional convenience of dosing, storage, transportation, and distribution.
3. They avoid the problems associated with either the aqueous noisome dispersion, such as problems of physical stability, aggregation, fusion, and leakage.
4. Proniosomes also avoid problems associated with liposomes like degradation by hydrolysis or oxidation as well as sedimentation, aggregation or fusion during storage.
5. Proniosomes not only offer a promising means of drug delivery, but also could enhance the recovery rate of the skin barrier. [20]
Clinical Applications
Drug targetig
Applications in cardiology
Proniosomes are used as carriers for the transdermal delivery of captopril for the treatment of hypertension. The study shows that the proniosomal system causes extended release of the drug in the body. Encapsulation of the drug is carried out using Sorbitan esters, Cholesterol and lecithin. [22]
Application in diabetes
Skin permeation mechanism of furesamide proniosomes is performed in which span, soya, lecithin, diacetyl phosphate, and cholesterol were used. Over all findings suggest that the proniosomes serve as non-invasive delivery of furesamide. [23]
Hormonal therapy
Work had been performed on proniosome based transdermal delivery of levonorgestrel the emergency contraceptive. The structure of the niosome was liquid crystalline compact hybrid. The system was tested for particle size, encapsulation efficiency, stability study, in vivo and in vitro study. Bioassay for progestational activity was also performed. It included endometrial assay and blockade of development of corpora lutea. [24]
Delivery of peptide drugs
Oral peptide drug delivery has a drawback of bypassing the enzymes, which would breakdown the peptide and protein bonds. Niosomes were used to successfully protect the peptides from gastrointestinal peptide breakdown. Oral delivery of vasopressin derivative entrapped in niosomes showed that entrapment of the drug significantly increased the stability of the peptide. [21]
Uses in studying immune response
Immune response was studied using niosomes due to their immunological selectivity, low toxicity and greater stability. Niosomes and proniosomes are being used to study the nature of the immune response provoked by antigens. [1,14]
Niosomes as carriers for hemoglobin
Blood has many carrier proteins present in it. niosomescan be used as carriers for hemoglobin within the blood. The niosomal or the proniosomal vesicle is permeable to oxygen and hence it acts as a carrier for hemoglobin in patients.
Other applications
Sustained release
Sustained release action of niosomes can be applied to drugs with low therapeutic index and low water solubility since those could be maintained in the circulation via niosomal encapsulation. [25]
Localized drug action
Drug delivery through niosomes is one of the approaches to achieve localized drug action, since their size and low penetrability through epithelium and connective tissue keeps the drug localized at the site of administration. Localized drug action results in enhancement of efficacy of potency of the drug and at the same time reduces its systemic toxic effects e.g., Anti-monials encapsulated within niosomes are taken up by mononuclear cells resulting in localization of drug, increase in potency and hence decrease both in dose and toxicity. The evolution of niosomal drug delivery technology is still at an infancy stage, but this type of drug delivery system has shown promise in cancer chemotherapy and anti-leishmanial therapy [Table 2]. [26]
| Drug | Category | Result (s) | Ref (s) |
|---|---|---|---|
| Glipizide | Oral rapid and short acting anti-diabetic | Glipizide will be successfully entrapped within the bilayer of the vesicles with high entrapment efficiency, and is a promising approach to sustain the drug release for an extended period of time and by that reducing the side-effects related to gastric irritation | 21 |
| Ketorolac | NSAID | Fusion of the vesicles with the intercellular lipid of the stratum corneum and direct transfer of drug from vesicles to the skin or the penetration enhancement effect of the non-ionic surfactants may contribute to the mechanism of drug permeation enhancement by proniosomal formulations | 27 |
| Piroxicam | NSAID | Permeation of piroxicam from proniosome based reservoir type transdermal gel formulation across excised rat abdominal skin was investigated using keshery-chein diffusion cell, there was a considerable improvement in flux over the control gel formulation | 14 |
| Aceclofenac | NSAID | The polynomial equation and contour plots developed by using central composite design allowed to prepare Proniosomes with optimum characteristic | 28 |
| Haloperidol | Antipsychotic | The formulation with single surfactant increased the permeation of drug more than those with a mixture of surfactants | 29 |
| Ibuprofen | NSAID | Proniosomes derived niosomes are superior in their ability to release the drug at a constant rate | 30 |
| Pseudo- Ceramide | Anti-wrinkle | It is a liquid delivery, applied topically at the site to show the action | 31 |
| Amphotericine | Anti-bacterial | Physical stability of preparations can be increased, vacuum is required during preparation and storage to prevent oxidation of phospholipids | 31 |
| Captopril | Anti-hypertensive | This is injected transdermally, it shows targeted drug action reducing blood pressure | 31 |
| Frusemide | Diuritec | This is injected transdermally, reduces the glucose levels | 31 |
| Aceclofenac | Antiinflammatory | The entrapped aceclofenac within niosomes was determined after removing the unentrapped drug by dialysis. The noisome dispersion acts specifically on inflammatory sites and thereby decreases the inflammation like rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis | 32 |
| Carbamazepine | Anti-epileptic | The CBZ is incorporated in bilayers of niosomal vesicles and is release with a rapid rate in bio environment. Since gastrointestinal mucosal barrier is negatively charged a possible electrostatic interaction between positively charged surface ligand and mucosa, keeps vesicles firmly adhere to the mucosa as a result CBZ absorption may be envisaged. Since CBZ absorption is poor and erratic, the proniosomal system developed by them might pave the way to resolve this issue there by offering better therapeutic benefits | 33 |
| Valsartan | Angiotensin II inhibitor | Here, most of the vesicles are well identified, spherical, and discreet with sharp boundaries having large internal aqueous space. The formulation is potentially used for the delivery of drug, an increase in cholesterol content has also been found to result in an increase in micro viscosity of membrane leading to more rigidity of the bilayers | 15 |
CBZ: Carbamazepine, NSAID: Non steroidal anti inflammatory drugs
Table 2: Some of the research work carriedout on proniosomes as drug carriers
Conclusion
Lately, there has been a magnanimous growth in drug delivery technologies, of which proniosomes are one of the sterile drug delivery systems, that are highly used in cancer therapies.
From the above article, it is concluded that the concept of incorporating the drug into niosomes for a better targeting of the drug at appropriate tissue destination is widely accepted by researchers and academicians. Proniosomes derived niosomes represent a promising drug delivery module. They are known to avoid many of the problems associated with either the aqueous noisome dispersion as problems of physical stability such as aggregation, fusion, and leakage. They provide additional convenience of transportation, distribution, storage and dosing. Proniosomes not only offer a promising means of drug delivery, but also could enhance the recovery rate of the skin barrier.
Proniosomes represent a promising drug delivery technologies and much research has to be inspired in this to filter out all the potential in this novel drug delivery systems.
All this make proniosmes ,that is “dry niosomes,” a promising industrial and research product.
References
- Kakr R, Rao R, Goswami A, Nanda S, Saroha K. Proniosomes: An emerging vesicular system in drug delivery and cosmetics. Der Pharmacia Lettre 2010;2:227-39.
- Walve JR, Rane BR, Gujrathi NA. Proniosomes: A surrogate carrier for improved transdermal drug delivery system. Int J Res Ayurveda Pharm 2011;2:743-50.
- Dua JS, Rana AC, Bhandari AK. Liposomes methods of preparation and applications. Int J Pharm Stud Res 2012;3:14-20.
- Daemen T, de Mare A, Bungener L, de Jonge J, Huckriede A, Wilschut J. Virosomes for antigen and DNA delivery. Adv Drug Deliv Rev 2005;57:451-63.
- Mujoriya RZ, Dhamandeb K, Bodla RB. Niosomal drug delivery systems-a review. Int J Appl Pharm 2011;3:7-10.
- Akhilesh D, Prabhu P, Faishal G. Comparative study of carriers used in proniosomes. Int J Pharm Chem Sci 2012;4:307-14.
- Rai K, Gupta Y, Jain A, Jain SK. Transfersomes: Self-optimizing carriers for bioactives. PDA J Pharm Sci Technol 2008;62:362-79.
- Kish-Trier E, Hill CP. Structural biology of the proteasome. Annu Rev Biophys 2013;42:1.1-1.21.
- Lankalapalli S, Damuluri M. Sphingosomes: Applications in targeted drug delivery. Int J Pharm Chem Biol Sci 2012;2:507-16.
- Sprott GD, Sad S, Fleming LP, Dicaire CJ, Patel GB, Krishnan L. Archaeosomes varying in lipid composition differ in receptor-mediated endocytosis and differentially adjuvant immune responses to entrapped antigen. Archaea 2003;1:151-64.
- Rattanapak T, Young K, Rades T, Hook S. Comparative study of liposomes, transfersomes, ethosomes and cubosomes for transcutaneous immunisation: Characterisation and in vitro skin penetration. J Pharm Pharmacol 2012;64:1560-9.
- Mishra A, Kapoor A, Bhargava S. Proniosoml gel as a carrier for improved transdermal drug delivery. Asian J Pharm Life Sci 2011;1:370-9.
- Sankar V, Ruckmani K, Durga S, Jailani S. Proniosomes as drug carriers. Pak J Pharm Sci 2010;23:103-7.
- Chandra A, Sharma PK. Proniosome based drug delivery system of piroxicam. Afr J Pharm Pharmacol 2008;2:184-90.
- Kakkar R, Rao R, Kumar DN. Formulation and characterisation of valsartan proniosomes. Maejo Int J Sci Technol 2011;5:146-58.
- Pavala Rani N, Suryaprakashand TN, Senthamarai R. Formulation and evaluation of rifampicin and gatifl oxacinniosomes on logarithmicphase cultures of mycobacterium tuberculosis. Int J Pharm Biol Sci 2010;1:379-86.
- Keservani RK, Sharma AK, Ayaz MD. Novel drug delivery system for the vesicular delivery of drug by the niosomes. Int J Res Control Release 2011;1:1-8.
- Kapil S, Rao R, Saini V. Preparation and evaluation of lornoxicamniosomal gel. Int Res J Pharm 2012;3:378-83.
- Kumar GP, Rao PR. Nonionic surfactant vesicular systems for effective drug delivery – An overview. Acta Pharm Sinica B. 2011;1:208-19.
- Arunothayanun P, Bernard MS, Craig DQ, Uchegbu IF, Florence AT. The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. Int J Pharm 2000;201:7-14.
- Akhilesh D, Bini KB, Kamath JV. Comparative study of carriers used in proniosomes. Int J Pharm Chem Sci 2012;3:6-12.
- Gupta A, Prajapathi S, BalaMurugan K. Design and development of a proniosomal trandermal drug delivery system of captopril. Trop J Pharm Res 2007;6:687-93.
- Azeem A, Ahmad FJ, Talegaonkar S. Exploration of skin permeation mechanism of frusemide with proniosomes. Pharmazie 2009;64:735-40.
- Vora B, Khopade AJ, Jain NK. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Release 1998;54:149-65.
- Biju SS, Talegaonkar S, Misra PR. Vesicular systems: Anoverview. Indian J Pharm Sci 2006;68:141-57.
- Oommen E, Tiwari SB, Udupa N. Niosome entrapped B-cyclodextrin methotrexate complex as a drug delivery system. Indian J Pharm 1999;31:279-84.
- Alsarra IA, Bosela AA, Ahmed SM, Mahrous GM. Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur J Pharm Biopharm 2005;59:485-90.
- Tank CJ, Borkhataria CH, Baria AH. Formulation and evaluation of aceclofenac loaded maltodextrin based proniosomes. Int J Chem Technol Res 2009;1:567-73.
- Azarbayjani AF, Tan EH, Chan YW. In vitro drug release of niosomes prepared from various proniosomal haloperidol by proniosomal formulations with non-ionic surfactants. Biol Pharm Bull 2009;32: 1453-8.
- Rhodes DG. Proniosomes a novelcarrier preparation. Int J Pharm 1999;185:23-35.
- Duttshukla N, Tiwari M. Proniosomal drug delivery systems-clinical applications. Int J Res Pharm Biomed Sci 2011;29:880-7.
- Solanki A, Parikh J, Parikh R. Preparation, characterisation, optimization, and stability studies of aceclofenac proniosomes. Int J Pharm Res 2008;7:237-46.
- Goud BA, Raju J, Rambhau D. Improved oral absorption of carbamazepine from sorbiton monolaurate based proniosome systems containing charged surface ligands. Int J Biol Pharm Res 2012;3:37-42.
Keywords
Characterization, clinical applications, niosomes, proniosomes, targeted drug action
Introduction
In recent times, no single drug delivery system fulfills all the criteria, but attempts have been made through novel approaches. Many novel approaches emerged covering various routes of administration, to achieve either controlled or targeted delivery. The prime aim of novel drug delivery is maintenance of the constant and effective drug level in the body and minimizing the side-effects and it also localizes the drug action by targeting the drug delivery by using drug carriers.
Vesicular drug delivery is one of the approaches, which encapsulate the drug e.g.: Liposomes, niosomes, transferosomes, pharmacosomes, and provesicles such as proniosomes and proliposomes. Advantages of liposomes and niosomes over other conventional dosage forms are their particulate nature, which act as a drug reservoir. Few modifications can also be carried out in order to adjust the pattern and the drug release. It was also found out that modified vesicles had properties that successfully delivered drugs into deeper layers of the skin. [1]
From early 1980s, proniosomes have gained wide attention by researchers for their use as drug targeting agents and drug carriers to have a variety of merits while avoiding demerits associated with the conventional form of drugs. Niosomes are water soluble carrier particles, and these are dried to form a niosomal dispersion on brief agitation in hot aqueous media. This dehydrated product is called proniosomes. The resulting niosomes are very correlative to conventional niosomes and of higher size uniformity. The proniosomal approach reduces the problems associated with dry, freeflowing product, which is more stable during the storage and sterilization. The proniosomes are a versatile delivery system because of the ease of distribution, measuring, transfer, and storage. [2]
Proniosomes were studied as alternatives to liposomes and other carrier systems for entrapping both polar and nonpolar or hydrophobic and hydrophilic drugs. The additional merits with proniosomes are low toxicity owing to non-ionicnature, no requirement of special precautions and conditions for formulation and preparations. In addition, it is the simple method for the routine and large scale production of proniosomes without the use of undesirable solvents. However, stability is a main concern in the advancement of any formulation and even proniosomes have advantages as drug carriers, such as cost productivity, chemically stability in comparision to liposomes. They also minimize problems of physical stability such as fusion, leakage, sedimentation, and aggregation on storage.
All these advantages of dry niosomes often termed as proniosomes have made them a promising industrial product.
Structure
Proniosomes are microscopic lamellar structures. They combine a non-ionic surfactant of the alkyl or dialkyl polyglycerol ether class and cholesterol followed by hydration in aqueous media. The surfactant molecule direct themselves such that the hydrophilic ends of the non-ionic surfactant orient outward, while the hydrophobic ends are in the opposite direction to form the bilayer. Like liposomes proniosomes are also made up of a bilayer. In proniosomes the bilayer is made of non-ionic surface active agents. [2]
On the basis of method of preparation proniosomes are unilamellar or multi-lmellar. The niosome is made of a surfactant bilayer with its hydrophilic ends exposed on the outside and inside of the vesicles while the hydrophobic chains face each other within the bilayer. Hence the vesicle holds hydrophilic drugs within the space enclosed in the vesicle and the hydrophobic drugs are embedded within the bilayer.
Types of Vesicular Drug Delivery Systems
1. Liposomes
2. Virosomes
3. Niosomes
4. Proniosomes
5. Transferosomes
6. Proteasomes
7. Sphingosomes
8. Archaesome
9. Ethosomes
Liposomes
Liposomes are artificially prepared vesicle composed of a bilayer of lipids. These can be used as a vehicle for administration of nutrients and pharmaceutical drugs. Liposomes can be prepared by disrupting biological membranes. They are composed of natural phospholipids and may also contain mixed lipid chains with surfactant properties. [3]
Virosomes
Virosomes are drug or vaccine delivery mechanism consisting of unilamellar phospholipid membrane, which is either a mono or bi-layer vesicle incorporating virus derived proteins to allow the virosomes to fuse with target cells. [4]
Niosomes
A niosome is a non-ionic surfactant-based liposome. Niosomes are formed mostly by cholesterol incorporation as an excipient. Other excipients can also be used. Niosomes have more penetrating capability than the previous preparations of emulsions. They are structurally similar to liposomes in having a bilayer; however, the materials used to prepare niosomes make them more stable and thus niosomes offer many more advantages over liposomes. [5]
Proniosomes
Proniosomes are dry formulation of water soluble carrier particles that are coated with surfactant. They are rehydrated to form niosomal dispersion immediately before use on agitation in hot aqueous media within minutes. Proniosomes are physically stable during storage and transport. Drug encapsulated in vesicular structure of proniosomes prolong the existence of drug in the systematic circulation and enhances the penetration into target tissue and reduce toxicity. [6]
Transferosomes
A transferosome carrier is an artificial vesicle designed to be such as a cell vesicle or a cell engaged in exocytosis and thus suitable for controlled and potentially targeted drug delivery. [7]
Proteasomes
Proteasomes are cytoplasmic organelle, composed of a cylindric core particle bound by two regulatory particles at each end, responsible for degrading endogenous proteins. Proteins to be destroyed are recognized by proteasomes because of the presence of ubiquitin conjugated to the targeted protein’s lysine residue. [8]
Sphingosomes
Sphingosomes is bilayered vesicles in which an aqueous volume is entirely enclosed by membrane lipid bilayer mainly composed of natural or synthetic sphingolipid. Sphingosomes solve the major drawback of vesicle system (liposomes, niosomes) such as less stability, less in vivo circulation time, low tumor loading efficacy in case of cancer therapy. Sphingosomes are clinically used delivery system for chemotherapeutic agent, biological macromolecule and diagnostics. Owing to flexibility in size and composition, different types of sphingosomes have been developed. [9]
Archaesome
Archaeosomes are liposomes made from the polar ether lipids of Archaea. These lipids are unique and distinct in structure from the ester lipids found in Eukarya and Bacteria. [10]
Ethosomes
Ethosomes are soft, malleable vesicles tailored for enhanced delivery of active agents. It has been shown that the physicochemical characteristics of ethosomes allow this vesicular carrier to transport active substances more efficaciously through the stratum corneum into the deeper layers of the skin than conventional liposomes. [11]
Action of Proniosomes
Proniosomes show their action after they are converted to niosomes on hydration. Proniosomes → Niosomes (on hydration). The hydration may occur by the addition of aqueous solvents. Proniosomes can entrap both hydrophilic and lipophilic drugs [Table 1]. [12]
| Materials | Specification | Action |
|---|---|---|
| Span and tween | Surfactants | Maintains HLB level |
| Cholesterol and | Membrane | Cholesterol: Influences the |
| lecithin | stabilizers | stability and permeability of |
| vesicles | ||
| Lecithin: Penetration enhancer | ||
| Maltodextrin, lactose, | Carriers | Holds the drug |
| sorbitol, mannitol | ||
| Methanol, chloroform, | Organic | Influence on vesicle size and |
| ethyl alcohol | solvents | permeability of drug |
HLB: Hydrophilic lipophilic balance
Table 1: Different materials used and their action in the preparation of proniosomes
Types of Proniosomes
According to the type of carrier and method of preparation of proniosomes they are of two types.
Dry granular proniosomes
1. Sorbitol based proniosomes
2. Maltodextrin based proniosomes
Sorbitol based proniosomes is a dry formulation that involves sorbitol as a carrier, which is further coated with non-ionic surfactant and is used as a noisome within minutes by the addition of hot water followed by agitation.. [1]
Maltodextrin based proniosomes are prepared by fast slurry method. [1]
Liquid crystalline proniosomes
This type of proniosomes are reservoirs for transdermal delivery of the drug. The transdermal patch involves an aluminum foil as a baking material along with a plastic sheet. Proniosomal gel is spread evenly on the circular plastic sheet followed by covering with a nylon mesh. [1]
Preparation of Proniosomes
The proniosomes consist of a number of ingredients such as the non-ionicsurfactant, cholesterol orlecithin being the main ingredient. Some of the methods, which were reported for the preparation of proniosomes are as follows:
1. Hand shaking method
2. Slurry method
3. Slow spray coating method
Hand shaking method
The mixture of vesicles forming ingredients such as cholesterol and surfactants are dissolved in ether, methanol or chloroform in a round bottom flask. The organic solvent evaporates at room temperature (20°C) in the rotary evaporator leaving a thin layer of solid mixture deposited on the walls of the round bottomed flask. The dried surfactant film can be rehydrated with the aqueous phase at 0-60°C with little agitation. This process produces typical multi-lamellarniosomes. [2]
Slurry method
A 250 μmol stock solution of surfactant and membrane stabilizer was prepared in chloroform:methanol (2:1) solution. A definite volume of stock solution and drug dissolved in chloroform:methanol (2:1) solution was added to a 100 ml round bottom flask containing the carrier marterial. Additional organic solvent solution added to form a slurry if lower surfactant loading occurs. The flask was attached to a rotary flash evaporator, which evaporates solvent at 60 -70 rpm, a temperature was 45 ± 2°C, and a reduced pressure of 600 mmHg until the mass in the flask had become a dry, free flowing product. These materials were dried in a desiccator overnight at room temperature under vacuum. This dry preparation is referred to as “proniosomes” and was used for preparations and for further study on powder properties. These final products ,that is proniosomes were stored in a tightly closed container at refrigerator temperature until further evaluation. [2]
Slow spray coating method
A 100 ml round bottom flask containing desired amount of carrier can be attached to rotary evaporator. The evaporator has to be evacuated and rotating flask can be rotated in a water bath under vacuum at 65-70oC for 15-20 min. This process is repeated until all of the surfactant solution has been applied. The evaporation should be continued until the powder becomes completely dry. [12,13]
Characterization of Proniosomes
Evaluation studies are further carried out for the prepared proniosomes in order to find out the
• Measurement of angle of repose
• Scanning electron microscopy (SEM)
• Optical microscopy
• Measurement of vesicle size
• Drug content
• Entrapment effi ciency
• In-vivo release studies
• Stability studies
Measurement of angle of repose
The angle of repose of dried proniosomes was measured by funnel method and cylinder method.
Funnel method
The funnel, which was fixed at a position and the proniosomal powder was poured into it so that the outlet orifice of the funnel is 10 cm above the level of surface. The powder flowed down from the funnel to form a cone on the surface and then angle of repose was further calculated by measuring the height of the cone and the diameter of its base.
Cylinder method
The proniosomes powder was poured into a cylinder, which was fixed at a position so that the outlet orifice of the cylinder is 10 cm above the level of surface. The powder flowed down in the cylinder to form a cone on the surface. The angle of repose was further calculated by measuring the height of the cone and the diameter of its base. [12]
Angle of repose is calculated by the below equation
θ = Tan-1 × (h/r)
SEM
Particle size of proniosomes is a factor of prime importance. The surface morphology and size distribution of proniosomes were studied by SEM. A double-sided tape that was affixed on aluminum stubs and the proniosomal powder was spread on it. The aluminum stub was placed in a vacuum chamber of scanning electron microscope (XL 30 ESEM with EDAX, Philips, Netherlands). The morphological characterization of the samples was observed using a gaseous secondary electron detector (working pressure of 0.8 torr, acceleration voltage-30.00 KV) XL 30, (Philips, Netherlands). [15]
Optical microscopy
The niosomes were mounted on glass slides and viewed under a microscope (Medilux-207RII, Kyowa-G etner, Ambala, India). The microscope has a magnification of ×1200 used for morphological observation after sufficient dilution. The photomicrograph of the preparation was obtained from the microscope by using a digital Single lens reflex (SLR) camera. [15]
Measurement of vesicle size
The vesicle dispersions were diluted about 100 times in the same medium, which was used for their preparation. Vesicle size was measured on a particle size analyzer. The apparatus consist of a He-Ne laser beam of 632.8 nm focused with a minimum power of 5Mw using a Fourier lens (R-5) to a point at the center of multi-element detector and a small volume sample holding cell. The samples were stirred with a stirrer before determining the vesicle size. [16]
Drug content
Proniosomes equivalent to 100 mg were taken in a standard volumetric flask. They were lysed with 50 ml methanol by shaking for 15 min. The solution was diluted to 100 ml with methanol. Then 10 mlof this solution was diluted to 100 ml with saline phosphate buffer at certain pH. Aliquots were withdrawn and absorbance was measured at a certain wavelength and drug content was further calculated from the calibration curve. [17]
Entrapment effificiency
Separation of unentrapped drug from the niosomal suspension was carried out by exhaustive dialysis method and centrifugation method. Theniosomal suspension was taken into a dialysis tube to which osmotic cellulose membrane was securely attached to one side, the dialysis tube was suspended in 100 ml saline buffer at certain pH, which was stirred on a magnetic stirrer. The niosomal suspension and the unentrapped drug were seperated into the medium through osmotic cellulose membrane. After 6 h of exhaustive dialysis, optical density values were noted and the estimation of the entrapped drug was carried out by UV spectrophotometric method. Entrapment Efficiency was calculated using the formula. [18]
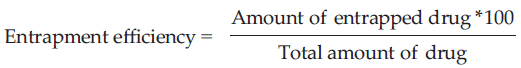
In vivo release studies
The release of the drug from the proniosomal formulations was determined using different techniques such as Franz diffusion cell, Keshary-Chien diffusion cell, Cellophane dialyzing membrane, United states pharmacopeia (USP) dissolution apparatus Type-1, spectrapor molecular porous membrane tubing. Drug release from proniosomes derived niosomal vesicles can follow any one or more of the following mechanisms; desorption from the surface of vesicles or diffusion of drug from bilayered membrane or a combined desorption and diffusion mechanisms. [19]
Stability studies
Stability studies were carried out by storing the prepared proniosomes at various temperature conditions such as refrigeration temperature (2°-8°C), room temperature (25° ± 0.5°C) and elevated temperature (45° ± 0.5°C) from a period of 1 month to 3 months. Drug content and variation in the average vesicle diameter were periodically monitored.
International conference on harmonization (ICH) guidelines suggests stability studies for the dry proniosome powders meant for reconstitution should be studied for accelerated stability at 40°C/75% relative humidity as per international climatic zones and climatic conditions (WHO, 1996). For long term stability studies the temperature is 25°C/60% RH for the countries in zone I and II and for the countries in zone III and IV the temperature is 30°C/65% Relative humidity (RH). Product should be evaluated for appearance, color, assay, pH preservative content, particulate matter, sterility, and pyrogenicity. [19]
Advantages of Proniosomes
1. Both the non-ionic surfactants and phospholipids in proniosomes can act as penetration enhancers and help in diffusion of the drug.
2. Proniosomes have higher advantages such as additional convenience of dosing, storage, transportation, and distribution.
3. They avoid the problems associated with either the aqueous noisome dispersion, such as problems of physical stability, aggregation, fusion, and leakage.
4. Proniosomes also avoid problems associated with liposomes like degradation by hydrolysis or oxidation as well as sedimentation, aggregation or fusion during storage.
5. Proniosomes not only offer a promising means of drug delivery, but also could enhance the recovery rate of the skin barrier. [20]
Clinical Applications
Drug targetig
Applications in cardiology
Proniosomes are used as carriers for the transdermal delivery of captopril for the treatment of hypertension. The study shows that the proniosomal system causes extended release of the drug in the body. Encapsulation of the drug is carried out using Sorbitan esters, Cholesterol and lecithin. [22]
Application in diabetes
Skin permeation mechanism of furesamide proniosomes is performed in which span, soya, lecithin, diacetyl phosphate, and cholesterol were used. Over all findings suggest that the proniosomes serve as non-invasive delivery of furesamide. [23]
Hormonal therapy
Work had been performed on proniosome based transdermal delivery of levonorgestrel the emergency contraceptive. The structure of the niosome was liquid crystalline compact hybrid. The system was tested for particle size, encapsulation efficiency, stability study, in vivo and in vitro study. Bioassay for progestational activity was also performed. It included endometrial assay and blockade of development of corpora lutea. [24]
Delivery of peptide drugs
Oral peptide drug delivery has a drawback of bypassing the enzymes, which would breakdown the peptide and protein bonds. Niosomes were used to successfully protect the peptides from gastrointestinal peptide breakdown. Oral delivery of vasopressin derivative entrapped in niosomes showed that entrapment of the drug significantly increased the stability of the peptide. [21]
Uses in studying immune response
Immune response was studied using niosomes due to their immunological selectivity, low toxicity and greater stability. Niosomes and proniosomes are being used to study the nature of the immune response provoked by antigens. [1,14]
Niosomes as carriers for hemoglobin
Blood has many carrier proteins present in it. niosomescan be used as carriers for hemoglobin within the blood. The niosomal or the proniosomal vesicle is permeable to oxygen and hence it acts as a carrier for hemoglobin in patients.
Other applications
Sustained release
Sustained release action of niosomes can be applied to drugs with low therapeutic index and low water solubility since those could be maintained in the circulation via niosomal encapsulation. [25]
Localized drug action
Drug delivery through niosomes is one of the approaches to achieve localized drug action, since their size and low penetrability through epithelium and connective tissue keeps the drug localized at the site of administration. Localized drug action results in enhancement of efficacy of potency of the drug and at the same time reduces its systemic toxic effects e.g., Anti-monials encapsulated within niosomes are taken up by mononuclear cells resulting in localization of drug, increase in potency and hence decrease both in dose and toxicity. The evolution of niosomal drug delivery technology is still at an infancy stage, but this type of drug delivery system has shown promise in cancer chemotherapy and anti-leishmanial therapy [Table 2]. [26]
| Drug | Category | Result (s) | Ref (s) |
|---|---|---|---|
| Glipizide | Oral rapid and short acting anti-diabetic | Glipizide will be successfully entrapped within the bilayer of the vesicles with high entrapment effi ciency, and is a promising approach to sustain the drug release for an extended period of time and by that reducing the side-effects related to gastric irritation | 21 |
| Ketorolac | NSAID | Fusion of the vesicles with the intercellular lipid of the stratum corneum and direct transfer of drug from vesicles to the skin or the penetration enhancement effect of the non-ionic surfactants may contribute to the mechanism of drug permeation enhancement by proniosomal formulations | 27 |
| Piroxicam | NSAID | Permeation of piroxicam from proniosome based reservoir type transdermal gel formulation across excised rat abdominal skin was investigated using keshery-chein diffusion cell, there was a considerable improvement in fl ux over the control gel formulation | 14 |
| Aceclofenac | NSAID | The polynomial equation and contour plots developed by using central composite design allowed to prepare Proniosomes with optimum characteristic | 28 |
| Haloperidol | Antipsychotic | The formulation with single surfactant increased the permeation of drug more than those with a mixture of surfactants | 29 |
| Ibuprofen | NSAID | Proniosomes derived niosomes are superior in their ability to release the drug at a constant rate | 30 |
| Pseudo- Ceramide | Anti-wrinkle | It is a liquid delivery, applied topically at the site to show the action | 31 |
| Amphotericine | Anti-bacterial | Physical stability of preparations can be increased, vacuum is required during preparation and storage to prevent oxidation of phospholipids | 31 |
| Captopril | Anti-hypertensive | This is injected transdermally, it shows targeted drug action reducing blood pressure | 31 |
| Frusemide | Diuritec | This is injected transdermally, reduces the glucose levels | 31 |
| Aceclofenac | Antiinfl ammatory | The entrapped aceclofenac within niosomes was determined after removing the unentrapped drug by dialysis. The noisome dispersion acts specifi cally on infl ammatory sites and thereby decreases the infl ammation like rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis | 32 |
| Carbamazepine | Anti-epileptic | The CBZ is incorporated in bilayers of niosomal vesicles and is release with a rapid rate in bio environment. Since gastrointestinal mucosal barrier is negatively charged a possible electrostatic interaction between positively charged surface ligand and mucosa, keeps vesicles fi rmly adhere to the mucosa as a result CBZ absorption may be envisaged. Since CBZ absorption is poor and erratic, the proniosomal system developed by them might pave the way to resolve this issue there by offering better therapeutic benefi ts | 33 |
| Valsartan | Angiotensin II inhibitor | Here, most of the vesicles are well identifi ed, spherical, and discreet with sharp boundaries having large internal aqueous space. The formulation is potentially used for the delivery of drug, an increase in cholesterol content has also been found to result in an increase in micro viscosity of membrane leading to more rigidity of the bilayers | 15 |
CBZ: Carbamazepine, NSAID: Non steroidal anti inflammatory drugs
Table 2: Some of the research work carriedout on proniosomes as drug carriers
Conclusion
Lately, there has been a magnanimous growth in drug delivery technologies, of which proniosomes are one of the sterile drug delivery systems, that are highly used in cancer therapies.
From the above article, it is concluded that the concept of incorporating the drug into niosomes for a better targeting of the drug at appropriate tissue destination is widely accepted by researchers and academicians. Proniosomes derived niosomes represent a promising drug delivery module. They are known to avoid many of the problems associated with either the aqueous noisome dispersion as problems of physical stability such as aggregation, fusion, and leakage. They provide additional convenience of transportation, distribution, storage and dosing. Proniosomes not only offer a promising means of drug delivery, but also could enhance the recovery rate of the skin barrier.
Proniosomes represent a promising drug delivery technologies and much research has to be inspired in this to filter out all the potential in this novel drug delivery systems.
All this make proniosmes ,that is “dry niosomes,” a promising industrial and research product.
References
- Kakr R, Rao R, Goswami A, Nanda S, Saroha K. Proniosomes: An emerging vesicular system in drug delivery and cosmetics. Der Pharmacia Lettre 2010;2:227-39.
- Walve JR, Rane BR, Gujrathi NA. Proniosomes: A surrogate carrier for improved transdermal drug delivery system. Int J Res Ayurveda Pharm 2011;2:743-50.
- Dua JS, Rana AC, Bhandari AK. Liposomes methods of preparation and applications. Int J Pharm Stud Res 2012;3:14-20.
- Daemen T, de Mare A, Bungener L, de Jonge J, Huckriede A, Wilschut J. Virosomes for antigen and DNA delivery. Adv Drug Deliv Rev 2005;57:451-63.
- Mujoriya RZ, Dhamandeb K, Bodla RB. Niosomal drug delivery systems-a review. Int J Appl Pharm 2011;3:7-10.
- Akhilesh D, Prabhu P, Faishal G. Comparative study of carriers used in proniosomes. Int J Pharm Chem Sci 2012;4:307-14.
- Rai K, Gupta Y, Jain A, Jain SK. Transfersomes: Self-optimizing carriers for bioactives. PDA J Pharm Sci Technol 2008;62:362-79.
- Kish-Trier E, Hill CP. Structural biology of the proteasome. Annu Rev Biophys 2013;42:1.1-1.21.
- Lankalapalli S, Damuluri M. Sphingosomes: Applications in targeted drug delivery. Int J Pharm Chem Biol Sci 2012;2:507-16.
- Sprott GD, Sad S, Fleming LP, Dicaire CJ, Patel GB, Krishnan L. Archaeosomes varying in lipid composition differ in receptor-mediated endocytosis and differentially adjuvant immune responses to entrapped antigen. Archaea 2003;1:151-64.
- Rattanapak T, Young K, Rades T, Hook S. Comparative study of liposomes, transfersomes, ethosomes and cubosomes for transcutaneous immunisation: Characterisation and in vitro skin penetration. J Pharm Pharmacol 2012;64:1560-9.
- Mishra A, Kapoor A, Bhargava S. Proniosoml gel as a carrier for improved transdermal drug delivery. Asian J Pharm Life Sci 2011;1:370-9.
- Sankar V, Ruckmani K, Durga S, Jailani S. Proniosomes as drug carriers. Pak J Pharm Sci 2010;23:103-7.
- Chandra A, Sharma PK. Proniosome based drug delivery system of piroxicam. Afr J Pharm Pharmacol 2008;2:184-90.
- Kakkar R, Rao R, Kumar DN. Formulation and characterisation of valsartan proniosomes. Maejo Int J Sci Technol 2011;5:146-58.
- Pavala Rani N, Suryaprakashand TN, Senthamarai R. Formulation and evaluation of rifampicin and gatifl oxacinniosomes on logarithmicphase cultures of mycobacterium tuberculosis. Int J Pharm Biol Sci 2010;1:379-86.
- Keservani RK, Sharma AK, Ayaz MD. Novel drug delivery system for the vesicular delivery of drug by the niosomes. Int J Res Control Release 2011;1:1-8.
- Kapil S, Rao R, Saini V. Preparation and evaluation of lornoxicamniosomal gel. Int Res J Pharm 2012;3:378-83.
- Kumar GP, Rao PR. Nonionic surfactant vesicular systems for effective drug delivery – An overview. Acta Pharm Sinica B. 2011;1:208-19.
- Arunothayanun P, Bernard MS, Craig DQ, Uchegbu IF, Florence AT. The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether. Int J Pharm 2000;201:7-14.
- Akhilesh D, Bini KB, Kamath JV. Comparative study of carriers used in proniosomes. Int J Pharm Chem Sci 2012;3:6-12.
- Gupta A, Prajapathi S, BalaMurugan K. Design and development of a proniosomal trandermal drug delivery system of captopril. Trop J Pharm Res 2007;6:687-93.
- Azeem A, Ahmad FJ, Talegaonkar S. Exploration of skin permeation mechanism of frusemide with proniosomes. Pharmazie 2009;64:735-40.
- Vora B, Khopade AJ, Jain NK. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J Control Release 1998;54:149-65.
- Biju SS, Talegaonkar S, Misra PR. Vesicular systems: Anoverview. Indian J Pharm Sci 2006;68:141-57.
- Oommen E, Tiwari SB, Udupa N. Niosome entrapped B-cyclodextrin methotrexate complex as a drug delivery system. Indian J Pharm 1999;31:279-84.
- Alsarra IA, Bosela AA, Ahmed SM, Mahrous GM. Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur J Pharm Biopharm 2005;59:485-90.
- Tank CJ, Borkhataria CH, Baria AH. Formulation and evaluation of aceclofenac loaded maltodextrin based proniosomes. Int J Chem Technol Res 2009;1:567-73.
- Azarbayjani AF, Tan EH, Chan YW. In vitro drug release of niosomes prepared from various proniosomal haloperidol by proniosomal formulations with non-ionic surfactants. Biol Pharm Bull 2009;32: 1453-8.
- Rhodes DG. Proniosomes a novelcarrier preparation. Int J Pharm 1999;185:23-35.
- Duttshukla N, Tiwari M. Proniosomal drug delivery systems-clinical applications. Int J Res Pharm Biomed Sci 2011;29:880-7.
- Solanki A, Parikh J, Parikh R. Preparation, characterisation, optimization, and stability studies of aceclofenac proniosomes. Int J Pharm Res 2008;7:237-46.
- Goud BA, Raju J, Rambhau D. Improved oral absorption of carbamazepine from sorbiton monolaurate based proniosome systems containing charged surface ligands. Int J Biol Pharm Res 2012;3:37-42.

